Lateral Habenula Glutamatergic Neurons Modulate Isoflurane Anesthesia in Mice
- PMID: 33746711
- PMCID: PMC7969819
- DOI: 10.3389/fnmol.2021.628996
Lateral Habenula Glutamatergic Neurons Modulate Isoflurane Anesthesia in Mice
Abstract
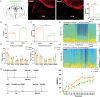
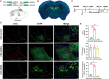
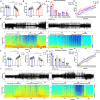
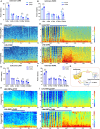
Since their introduction in the 1840s, one of the largest mysteries of modern anesthesia are how general anesthetics create the state of reversible loss of consciousness. Increasing researchers have shown that neural pathways that regulate endogenous sleep-wake systems are also involved in general anesthesia. Recently, the Lateral Habenula (LHb) was considered as a hot spot for both natural sleep-wake and propofol-induced sedation; however, the role of the LHb and related pathways in the isoflurane-induced unconsciousness has yet to be identified. Here, using real-time calcium fiber photometry recordings in vivo, we found that isoflurane reversibly increased the activity of LHb glutamatergic neurons. Then, we selectively ablated LHb glutamatergic neurons in Vglut2-cre mice, which caused a longer induction time and less recovery time along with a decrease in delta-band power in mice under isoflurane anesthesia. Furthermore, using a chemogenetic approach to specifically activate LHb glutamatergic neurons shortened the induction time and prolonged the recovery time in mice under isoflurane anesthesia with an increase in delta-band power. In contrast, chemogenetic inhibition of LHb glutamatergic neurons was very similar to the effects of selective lesions of LHb glutamatergic neurons. Finally, optogenetic activation of LHb glutamatergic neurons or the synaptic terminals of LHb glutamatergic neurons in the rostromedial tegmental nucleus (RMTg) produced a hypnosis-promoting effect in isoflurane anesthesia with an increase in slow wave activity. Our results suggest that LHb glutamatergic neurons and pathway are vital in modulating isoflurane anesthesia.
Keywords: glutamatergic; induction time; isoflurane; lateral habenula; nucleus; recovery time; rostromedial tegmental.
Copyright © 2021 Liu, Liu, Zhou, He, Zhang, Cai, Yuan, Luo, Zheng, Yu and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Lateral Hypothalamic Area Glutamatergic Neurons and Their Projections to the Lateral Habenula Modulate the Anesthetic Potency of Isoflurane in Mice.Neurosci Bull. 2021 Jul;37(7):934-946. doi: 10.1007/s12264-021-00674-z. Epub 2021 Apr 13. Neurosci Bull. 2021. PMID: 33847915 Free PMC article.
-
Lateral habenula-rostromedial tegmental nucleus circuit mediates inflammatory pain in mice.J Headache Pain. 2025 May 6;26(1):105. doi: 10.1186/s10194-025-02052-w. J Headache Pain. 2025. PMID: 40329200 Free PMC article.
-
Orexinergic innervations at GABAergic neurons of the lateral habenula mediates the anesthetic potency of sevoflurane.CNS Neurosci Ther. 2023 May;29(5):1332-1344. doi: 10.1111/cns.14106. Epub 2023 Feb 5. CNS Neurosci Ther. 2023. PMID: 36740262 Free PMC article.
-
Inhibition Within the Lateral Habenula-Implications for Affective Disorders.Front Behav Neurosci. 2021 Nov 26;15:786011. doi: 10.3389/fnbeh.2021.786011. eCollection 2021. Front Behav Neurosci. 2021. PMID: 34899206 Free PMC article. Review.
-
The Rostromedial Tegmental Nucleus: Anatomical Studies and Roles in Sleep and Substance Addictions in Rats and Mice.Nat Sci Sleep. 2020 Dec 24;12:1215-1223. doi: 10.2147/NSS.S278026. eCollection 2020. Nat Sci Sleep. 2020. PMID: 33380853 Free PMC article. Review.
Cited by
-
Effects of sevoflurane and isoflurane on acute myocardial infarction model establishment in mice.Biochem Biophys Rep. 2025 Apr 3;42:102000. doi: 10.1016/j.bbrep.2025.102000. eCollection 2025 Jun. Biochem Biophys Rep. 2025. PMID: 40236295 Free PMC article.
-
Claustrum modulates behavioral sensitivity and EEG activity of propofol anesthesia.CNS Neurosci Ther. 2023 Jan;29(1):378-389. doi: 10.1111/cns.14012. Epub 2022 Nov 9. CNS Neurosci Ther. 2023. PMID: 36353753 Free PMC article.
-
The lateral habenula contributes to regulation of body temperature.iScience. 2025 Jun 18;28(7):112923. doi: 10.1016/j.isci.2025.112923. eCollection 2025 Jul 18. iScience. 2025. PMID: 40678505 Free PMC article.
-
A Common Neuronal Ensemble in the Lateral Habenula Regulates Ciprofol Anesthesia in Mice.Pharmaceuticals (Basel). 2024 Mar 11;17(3):363. doi: 10.3390/ph17030363. Pharmaceuticals (Basel). 2024. PMID: 38543149 Free PMC article.
-
The activation of GABAergic neurons in the hypothalamic tuberomammillary nucleus attenuates sevoflurane and propofol-induced anesthesia in mice.Front Pharmacol. 2023 Jun 22;14:1153735. doi: 10.3389/fphar.2023.1153735. eCollection 2023. Front Pharmacol. 2023. PMID: 37426823 Free PMC article.
References
-
- Chastrette N., Pfaff D. W., Gibbs R. B. (1991). Effects of daytime and nighttime stress on Fos-like immunoreactivity in the paraventricular nucleus of the hypothalamus, the habenula, and the posterior paraventricular nucleus of the thalamus. Brain Res. 563 339–344. 10.1016/0006-8993(91)91559-j - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

