Analysis of Antidepressant Activity of Huang-Lian Jie-Du Decoction Through Network Pharmacology and Metabolomics
- PMID: 33746756
- PMCID: PMC7970346
- DOI: 10.3389/fphar.2021.619288
Analysis of Antidepressant Activity of Huang-Lian Jie-Du Decoction Through Network Pharmacology and Metabolomics
Abstract
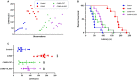
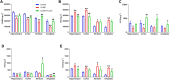
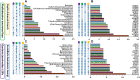
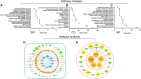
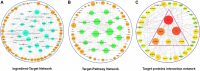
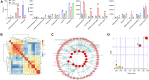
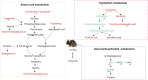
Depressive disorder is a common mental disorder characterized by depressed mood and loss of interest or pleasure. As the Herbal medicines are mainly used as complementary and alternative therapy for depression. This study aimed at exploring antidepressant activity of Huang-lian Jie-du Decoction (HLJDD), and evaluating active components and potential depression-associated targets. HLJDD was administered on chronic unpredictable mild stress-induced (CUMS) depressive mice. Behavior evaluation was performed through force swimming test (FST), novelty-suppressed feeding test (NSF), and open field test (OFT). Active components of HLJDD, potential targets, and metabolic pathways involved in depression were explored through systemic biology-based network pharmacology assay, molecular docking and metabonomics. FST assay showed that CUMS mice administered with HLJDD had significantly shorter immobility time compared with control mice. Further, HLJDD alleviated feeding latency of CUMS mice in NSFand increased moving distance and duration in OFT. In the following network pharmacology assay, thirty-eight active compounds in HLJDD were identified based on drug-like characteristics, and pharmacokinetics and pharmacodynamics profiles. Moreover, forty-eight molecular targets and ten biochemical pathways were uncovered through molecular docking and metabonomics. GRIN2B, DRD, PRKCA, HTR, MAOA, SLC6A4, GRIN2A, and CACNA1A are implicated in inhibition of depressive symptoms through modulating tryptophan metabolism, serotonergic and dopaminergic synaptic activities, cAMP signaling pathway, and calcium signaling pathway. Further network pharmacology-based analysis showed a correlation between HLJDD and tryptophan metabolism. A total of thirty-seven active compounds, seventy-six targets, and sixteen biochemical pathways were involved in tryptophan metabolism. These findings show that HLJDD acts on potential targets such as SLC6A4, HTR, INS, MAO, CAT, and FoxO, PI3K/Akt, calcium, HIF-1, and mTOR signaling pathways, and modulates serotoninergic and dopaminergic synaptic functions. In addition, metabonomics showed that tryptophan metabolism is the primary target for HLJDD in CUMS mice. The findings of the study show that HLJDD exhibited antidepressant effects. SLC6A4 and MAOA in tryptophan metabolism were modulated by berberine, baicalein, tetrahydroberberine, candicine and may be the main antidepressant targets for HLJDD.
Keywords: depression; huang-lian jie-du decoction; metabolomics; network pharmacology; tryptophan metabolism.
Copyright © 2021 Qu, Li, Heng, Qi, Ge, Ni, Yao, Guo, Yang, Cao, Zhang and Zhu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Bang-Andersen B., Ruhland T., Jorgensen M., Smith G., Frederiksen K., Jensen K. G., et al. (2011). Discovery of 1-[2-(2,4-dimethylphenylsulfanyl)phenyl]piperazine (Lu AA21004): a novel multimodal compound for the treatment of major depressive disorder. J. Med. Chem 54, 3206–3221. 10.1021/jm101459g - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

