Outer Membrane Vesicles of Gram-Negative Bacteria: An Outlook on Biogenesis
- PMID: 33746909
- PMCID: PMC7969528
- DOI: 10.3389/fmicb.2021.557902
Outer Membrane Vesicles of Gram-Negative Bacteria: An Outlook on Biogenesis
Abstract
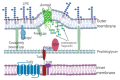
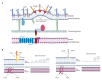
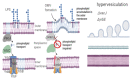
Outer membrane vesicles (OMVs) from Gram-negative bacteria were first described more than 50 years ago. However, the molecular mechanisms involved in biogenesis began to be studied only in the last few decades. Presently, the biogenesis and molecular mechanisms for their release are not completely known. This review covers the most recent information on cellular components involved in OMV biogenesis, such as lipoproteins and outer membrane proteins, lipopolysaccharide, phospholipids, quorum-sensing molecules, and flagella.
Keywords: LPS; OMVs biogenesis; PQS; bacterial vesicles; extracellular vesicles; flagellin; outer membrane vesicles; phospholipids.
Copyright © 2021 Avila-Calderón, Ruiz-Palma, Aguilera-Arreola, Velázquez-Guadarrama, Ruiz, Gomez-Lunar, Witonsky and Contreras-Rodríguez.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

