How Bacterial Adaptation to Cystic Fibrosis Environment Shapes Interactions Between Pseudomonas aeruginosa and Staphylococcus aureus
- PMID: 33746915
- PMCID: PMC7966511
- DOI: 10.3389/fmicb.2021.617784
How Bacterial Adaptation to Cystic Fibrosis Environment Shapes Interactions Between Pseudomonas aeruginosa and Staphylococcus aureus
Abstract
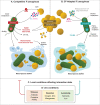
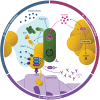
Pseudomonas aeruginosa and Staphylococcus aureus are the two most prevalent bacteria species in the lungs of cystic fibrosis (CF) patients and are associated with poor clinical outcomes. Co-infection by the two species is a frequent situation that promotes their interaction. The ability of P. aeruginosa to outperform S. aureus has been widely described, and this competitive interaction was, for a long time, the only one considered. More recently, several studies have described that the two species are able to coexist. This change in relationship is linked to the evolution of bacterial strains in the lungs. This review attempts to decipher how bacterial adaptation to the CF environment can induce a change in the type of interaction and promote coexisting interaction between P. aeruginosa and S. aureus. The impact of coexistence on the establishment and maintenance of a chronic infection will also be presented, by considering the latest research on the subject.
Keywords: P. aeruginosa; S. aureus; cystic fibrosis; evolution; interaction.
Copyright © 2021 Camus, Briaud, Vandenesch and Moreau.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Alhede M., Bjarnsholt T., Givskov M., Alhede M. (2014). Pseudomonas aeruginosa biofilms: mechanisms of immune evasion. Adv. Appl. Microbiol. 86 1–40. - PubMed
-
- Baldan R., Cigana C., Testa F., Bianconi I., De Simone M., Pellin D., et al. (2014). Adaptation of Pseudomonas aeruginosa in cystic fibrosis airways influences virulence of Staphylococcus aureus in vitro and murine models of co-infection. 3. PLoS One 9:e89614. 10.1371/journal.pone.0089614 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

