The Aryl Hydrocarbon Receptor as a Modulator of Anti-viral Immunity
- PMID: 33746961
- PMCID: PMC7973006
- DOI: 10.3389/fimmu.2021.624293
The Aryl Hydrocarbon Receptor as a Modulator of Anti-viral Immunity
Abstract
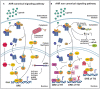
The aryl hydrocarbon receptor (AHR) is a ligand-activated transcription factor, which interacts with a wide range of organic molecules of endogenous and exogenous origin, including environmental pollutants, tryptophan metabolites, and microbial metabolites. The activation of AHR by these agonists drives its translocation into the nucleus where it controls the expression of a large number of target genes that include the AHR repressor (AHRR), detoxifying monooxygenases (CYP1A1 and CYP1B1), and cytokines. Recent advances reveal that AHR signaling modulates aspects of the intrinsic, innate and adaptive immune response to diverse microorganisms. This review will focus on the increasing evidence supporting a role for AHR as a modulator of the host response to viral infection.
Keywords: DNA viruses; RNA viruses; aryl hydrocarbon receptor; host response; viral infections.
Copyright © 2021 Torti, Giovannoni, Quintana and García.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

