Neutrophils in the Pathogenesis of Rheumatoid Arthritis and Systemic Lupus Erythematosus: Same Foe Different M.O
- PMID: 33746988
- PMCID: PMC7969658
- DOI: 10.3389/fimmu.2021.649693
Neutrophils in the Pathogenesis of Rheumatoid Arthritis and Systemic Lupus Erythematosus: Same Foe Different M.O
Abstract
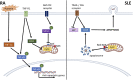
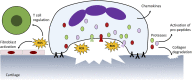
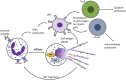
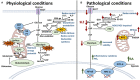
Dysregulated neutrophil activation contributes to the pathogenesis of autoimmune diseases including rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE). Neutrophil-derived reactive oxygen species (ROS) and granule proteases are implicated in damage to and destruction of host tissues in both conditions (cartilage in RA, vascular tissue in SLE) and also in the pathogenic post-translational modification of DNA and proteins. Neutrophil-derived cytokines and chemokines regulate both the innate and adaptive immune responses in RA and SLE, and neutrophil extracellular traps (NETs) expose nuclear neoepitopes (citrullinated proteins in RA, double-stranded DNA and nuclear proteins in SLE) to the immune system, initiating the production of auto-antibodies (ACPA in RA, anti-dsDNA and anti-acetylated/methylated histones in SLE). Neutrophil apoptosis is dysregulated in both conditions: in RA, delayed apoptosis within synovial joints contributes to chronic inflammation, immune cell recruitment and prolonged release of proteolytic enzymes, whereas in SLE enhanced apoptosis leads to increased apoptotic burden associated with development of anti-nuclear auto-antibodies. An unbalanced energy metabolism in SLE and RA neutrophils contributes to the pathology of both diseases; increased hypoxia and glycolysis in RA drives neutrophil activation and NET production, whereas decreased redox capacity increases ROS-mediated damage in SLE. Neutrophil low-density granulocytes (LDGs), present in high numbers in the blood of both RA and SLE patients, have opposing phenotypes contributing to clinical manifestations of each disease. In this review we will describe the complex and contrasting phenotype of neutrophils and LDGs in RA and SLE and discuss their discrete roles in the pathogenesis of each condition. We will also review our current understanding of transcriptomic and metabolomic regulation of neutrophil phenotype in RA and SLE and discuss opportunities for therapeutic targeting of neutrophil activation in inflammatory auto-immune disease.
Keywords: NETs; immunometabolism; low density granulocytes; neutrophils; rheumatoid arthritis; systemic lupus erythematosus.
Copyright © 2021 Fresneda Alarcon, McLaren and Wright.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

