Proteostatic Regulation of MEP and Shikimate Pathways by Redox-Activated Photosynthesis Signaling in Plants Exposed to Small Fungal Volatiles
- PMID: 33747018
- PMCID: PMC7973468
- DOI: 10.3389/fpls.2021.637976
Proteostatic Regulation of MEP and Shikimate Pathways by Redox-Activated Photosynthesis Signaling in Plants Exposed to Small Fungal Volatiles
Abstract
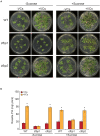
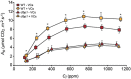
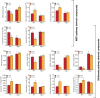
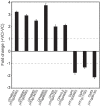
Microorganisms produce volatile compounds (VCs) with molecular masses of less than 300 Da that promote plant growth and photosynthesis. Recently, we have shown that small VCs of less than 45 Da other than CO2 are major determinants of plant responses to fungal volatile emissions. However, the regulatory mechanisms involved in the plants' responses to small microbial VCs remain unclear. In Arabidopsis thaliana plants exposed to small fungal VCs, growth promotion is accompanied by reduction of the thiol redox of Calvin-Benson cycle (CBC) enzymes and changes in the levels of shikimate and 2-C-methyl-D-erythritol 4-phosphate (MEP) pathway-related compounds. We hypothesized that plants' responses to small microbial VCs involve post-translational modulation of enzymes of the MEP and shikimate pathways via mechanisms involving redox-activated photosynthesis signaling. To test this hypothesis, we compared the responses of wild-type (WT) plants and a cfbp1 mutant defective in a redox-regulated isoform of the CBC enzyme fructose-1,6-bisphosphatase to small VCs emitted by the fungal phytopathogen Alternaria alternata. Fungal VC-promoted growth and photosynthesis, as well as metabolic and proteomic changes, were substantially weaker in cfbp1 plants than in WT plants. In WT plants, but not in cfbp1 plants, small fungal VCs reduced the levels of both transcripts and proteins of the stromal Clp protease system and enhanced those of plastidial chaperonins and co-chaperonins. Consistently, small fungal VCs promoted the accumulation of putative Clp protease clients including MEP and shikimate pathway enzymes. clpr1-2 and clpc1 mutants with disrupted plastidial protein homeostasis responded weakly to small fungal VCs, strongly indicating that plant responses to microbial volatile emissions require a finely regulated plastidial protein quality control system. Our findings provide strong evidence that plant responses to fungal VCs involve chloroplast-to-nucleus retrograde signaling of redox-activated photosynthesis leading to proteostatic regulation of the MEP and shikimate pathways.
Keywords: : Clp protease system; MEP pathway; PQC system; chloroplast-to-nucleus retrograde signaling; plant–microbe interaction; proteostatic regulation; redox regulation.
Copyright © 2021 Ameztoy, Sánchez-López, Muñoz, Bahaji, Almagro, Baroja-Fernández, Gámez-Arcas, De Diego, Doležal, Novák, Pěnčík, Alpízar, Rodríguez-Concepción and Pozueta-Romero.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Carretero-Paulet L., Ahumada I., Cunillera N., Rodríguez-Concepción M., Ferrer A., Boronat A., et al. (2002). Expression and molecular analysis of the Arabidopsis DXR gene encoding 1-deoxy-D-xylulose 5-phosphate reductoisomerase, the first committed enzyme of the 2-C-methyl-D-erythritol 4-phosphate pathway. Plant Physiol. 129 1581–1591. 10.1104/pp.003798 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

