Role of the long non-coding RNA HOTAIR/miR-126 axis in an in vitro psoriasis model
- PMID: 33747185
- PMCID: PMC7967857
- DOI: 10.3892/etm.2021.9878
Role of the long non-coding RNA HOTAIR/miR-126 axis in an in vitro psoriasis model
Abstract
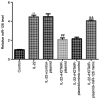
Psoriasis is a T-cell-mediated inflammatory skin disease that is characterized by excessive keratinocyte proliferation and persistent skin inflammation. Accumulating evidence suggests that long non-coding RNAs (lncRNAs) are dysregulated in a number of inflammatory conditions. In the present study, an in vitro psoriasis cell model was established. Human HaCaT keratinocytes were activated using the inflammatory factor IL-22. Briefly, HaCaT cells were starved in serum-free DMEM for 24 h and then stimulated with 100 ng/ml IL-22 in serum-free DMEM for 24 h. Previous research indicated that HOX transcript antisense RNA (HOTAIR) may participate in the development of psoriasis. First, reverse transcription-quantitative PCR (RT-qPCR) analysis was performed to detect HOTAIR expression. The results indicated that HOTAIR expression was reduced in IL-22-stimulated HaCaT cells. Subsequently, a dual-luciferase reporter assay was performed to verify the binding site between HOTAIR and microRNA (miR)-126. The RT-qPCR results indicated that miR-126 expression was increased in IL-22-stimulated HaCaT cells. Moreover, the effects of HOTAIR and miR-126 on IL-22-stimulated HaCaT cell proliferation and apoptosis were assessed. HaCaT cells were transfected with control-plasmid, HOTAIR-plasmid, HOTAIR-plasmid + mimic control or HOTAIR-plasmid + miR-126 mimic for 24 h. At 24 h post-transfection, the cells were stimulated with 100 ng/ml IL-22 for 24 h and experiments were conducted. IL-22 induced cell proliferation and suppressed apoptosis. However, HOTAIR-plasmid inhibited cell viability and induced apoptosis in IL-22-stimulated HaCaT cells. In addition, the western blotting results indicated that HOTAIR-plasmid increased cleaved caspase-3 expression and the cleaved caspase-3/caspase-3 ratio, whereas the HOTAIR-plasmid-mediated effects were reversed by miR-126 mimic. Collectively, the results of the present study demonstrated that the lncRNA-HOTAIR/miR-126 axis may be implicated in the regulation of psoriasis progression and may serve as a potential therapeutic target for psoriasis.
Keywords: interleukin-22-induced HaCaT cells; long non-coding RNA-HOX transcript antisense RNA; microRNA-126; psoriasis.
Copyright: © Zha et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
LncRNA MALAT-1 regulates the growth of interleukin-22-stimulated keratinocytes via the miR-330-5p/S100A7 axis.Autoimmunity. 2022 Feb;55(1):32-42. doi: 10.1080/08916934.2021.2001802. Epub 2021 Nov 11. Autoimmunity. 2022. PMID: 34761722
-
MiR-128-3p promotes hyperproliferation of keratinocytes and psoriasis-like inflammation by targeting SIRT1/HIF-1α.Arch Dermatol Res. 2025 Jan 4;317(1):165. doi: 10.1007/s00403-024-03669-8. Arch Dermatol Res. 2025. PMID: 39755881
-
Long non-coding RNA-HOTAIR promotes the progression of sepsis by acting as a sponge of miR-211 to induce IL-6R expression.Exp Ther Med. 2019 Nov;18(5):3959-3967. doi: 10.3892/etm.2019.8063. Epub 2019 Sep 27. Exp Ther Med. 2019. PMID: 31656541 Free PMC article.
-
Current Knowledge of Long Non-Coding RNA HOTAIR in Breast Cancer Progression and Its Application.Life (Basel). 2021 May 26;11(6):483. doi: 10.3390/life11060483. Life (Basel). 2021. PMID: 34073224 Free PMC article. Review.
-
Long Non-coding RNA HOTAIR in Central Nervous System Disorders: New Insights in Pathogenesis, Diagnosis, and Therapeutic Potential.Front Mol Neurosci. 2022 Jun 23;15:949095. doi: 10.3389/fnmol.2022.949095. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35813070 Free PMC article. Review.
Cited by
-
Identification of the potential biological target molecules related to primary open-angle glaucoma.BMC Ophthalmol. 2022 Apr 23;22(1):188. doi: 10.1186/s12886-022-02368-0. BMC Ophthalmol. 2022. PMID: 35461232 Free PMC article.
References
-
- Parisi R, Symmons DP, Griffiths CE, Ashcroft DM. Global epidemiology of psoriasis: A systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377–385. doi: 10.1038/jid.2012.339. Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
