BAFF receptor antibody for mantle cell lymphoma therapy
- PMID: 33747637
- PMCID: PMC7939563
- DOI: 10.1080/2162402X.2021.1893501
BAFF receptor antibody for mantle cell lymphoma therapy
Abstract
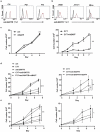
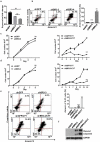
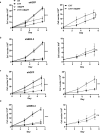
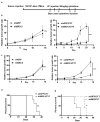
Mantle cell lymphoma (MCL) is an aggressive form of B cell non-Hodgkin's lymphoma and remains incurable under current treatment modalities. One of the main reasons for treatment failure is the development of drug resistance. Accumulating evidence suggests that B cell activating factor (BAFF) and BAFF receptor (BAFF-R) play an important role in the proliferation and survival of malignant B cells. High serum BAFF levels are often correlated with poor drug response and relapse in MCL patients. Our study shows that BAFF-R is expressed on both MCL patient cells and cell lines. BAFF-R knockdown leads to MCL cell death showing the importance of BAFF-R signaling in MCL survival. Moderate knockdown of BAFF-R in MCL cells did not affect its viability, but sensitized them to cytarabine treatment in vitro and in vivo, with prolonged mice survival. Anti-BAFF-R antibody treatment promoted drug-induced MCL cell death. Conversely, the addition of recombinant BAFF (rhBAFF) to MCL cells protected them from cytarabine-induced apoptosis. We tested the efficacy of a humanized defucosylated ADCC optimized anti-BAFF-R antibody in killing MCL. Our data show both in vitro and in vivo efficacy of this antibody for MCL therapy. To conclude, our data indicate that BAFF/BAFF-R signaling is crucial for survival and involved in drug resistance of MCL. Targeting BAFF-R using BAFF-R antibody might be a promising therapeutical strategy to treat MCL patients resistant to chemotherapy.
Keywords: Mantle Cell Lymphoma; adcc; baff; drug resistance; nk cells; therapy.
© 2021 The Author(s). Published with license by Taylor & Francis Group, LLC.
Conflict of interest statement
J.W is an employee at Novartis pharma. R.P is a scientific advisory board member of Luminary Therapeutics
Figures


References
-
- Reed DR, Portell CA.. Mantle cell lymphoma. Novel therapeutics for rare lymphomas. Springer, Cham. 2020;69–13.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
