MRI restoration using edge-guided adversarial learning
- PMID: 33747672
- PMCID: PMC7977797
- DOI: 10.1109/access.2020.2992204
MRI restoration using edge-guided adversarial learning
Abstract
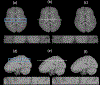
Magnetic resonance imaging (MRI) images acquired as multislice two-dimensional (2D) images present challenges when reformatted in orthogonal planes due to sparser sampling in the through-plane direction. Restoring the "missing" through-plane slices, or regions of an MRI image damaged by acquisition artifacts can be modeled as an image imputation task. In this work, we consider the damaged image data or missing through-plane slices as image masks and proposed an edge-guided generative adversarial network to restore brain MRI images. Inspired by the procedure of image inpainting, our proposed method decouples image repair into two stages: edge connection and contrast completion, both of which used general adversarial networks (GAN). We trained and tested on a dataset from the Human Connectome Project to test the application of our method for thick slice imputation, while we tested the artifact correction on clinical data and simulated datasets. Our Edge-Guided GAN had superior PSNR, SSIM, conspicuity and signal texture compared to traditional imputation tools, the Context Encoder and the Densely Connected Super Resolution Network with GAN (DCSRN-GAN). The proposed network may improve utilization of clinical 2D scans for 3D atlas generation and big-data comparative studies of brain morphometry.
Keywords: artifact correction; edge; generative adversarial network; image restoration; imputation; magnetic resonance imaging.
Figures





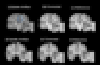
References
-
- Somasundaram K and Kalavathi P, “Analysis of Imaging Artifacts in MR Brain Images,” Orient. J Comput Sci Technol, vol. 5, no. (1), pp. 135–141, 2012.
-
- Stadler A and Ba-ssalamah A, “Artifacts in body MR imaging?: their appearance and how to eliminate them,” pp. 1242–1255, 2007. - PubMed
-
- Zhuo J and Gullapalli RP, “MR Artifacts, Safety, and Quality Control,” RadioGraphics, vol. 26, no. 1, pp. 275–297, 2006. - PubMed
-
- Welch EB, Felmlee JP, Ehman RL, and Manduca A, “Motion correction using the k-space phase difference of orthogonal acquisitions,” Magn. Reson. Med, vol. 48, no. 1, pp. 147–156, 2002. - PubMed
-
- Maclaren J, Herbst M, Speck O, and Zaitsev M, “Prospective motion correction in brain imaging: A review,” Magn. Reson. Med, vol. 69, no. 3, pp. 621–636, March. 2013. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
