Magneto-Based Synergetic Therapy for Implant-Associated Infections via Biofilm Disruption and Innate Immunity Regulation
- PMID: 33747747
- PMCID: PMC7967068
- DOI: 10.1002/advs.202004010
Magneto-Based Synergetic Therapy for Implant-Associated Infections via Biofilm Disruption and Innate Immunity Regulation
Abstract
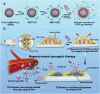
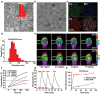
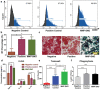
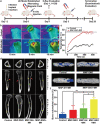
Implant-associated infections (IAIs) are a common cause of orthopedic surgery failure due to microbial biofilm-induced antibiotic-resistance and innate immune inactivation. Thus, the destruction of microbial biofilm plays a key role in reducing IAIs. Herein, first, a magneto-based synergetic therapy (MST) is proposed and demonstrated against IAIs based on biofilm destruction. Under an alternating magnetic field (AMF), CoFe2O4@MnFe2O4 nanoparticles (MNPs), with a rather strong magnetic hyperthermal capacity, can generate sufficient thermal effect to cause dense biofilm dispersal. Loosened biofilms provide channels through which nitrosothiol-coated MNPs (MNP-SNOs) can penetrate. Subsequently, thermosensitive nitrosothiols rapidly release nitric oxide (NO) inside biofilms, thus efficiently killing sessile bacteria under the magnetothermal effect of MNPs. More importantly, MNP-SNOs can trigger macrophage-related immunity to prevent the relapse of IAIs by exposing the infected foci to a consistent innate immunomodulatory effect. The notable anti-infection effect of this nanoplatform is also confirmed in a rat IAI model. This work presents the promising potential of combining magnetothermal therapy with immunotherapy, for the effective and durable control and elimination of IAIs.
Keywords: immunotherapy; implant‐associated infections; magnetic nanoparticles; magneto‐based synergetic therapy; nitric oxide.
© 2021 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
