Applications of Discrete Synthetic Macromolecules in Life and Materials Science: Recent and Future Trends
- PMID: 33747749
- PMCID: PMC7967060
- DOI: 10.1002/advs.202004038
Applications of Discrete Synthetic Macromolecules in Life and Materials Science: Recent and Future Trends
Abstract
In the last decade, the field of sequence-defined polymers and related ultraprecise, monodisperse synthetic macromolecules has grown exponentially. In the early stage, mainly articles or reviews dedicated to the development of synthetic routes toward their preparation have been published. Nowadays, those synthetic methodologies, combined with the elucidation of the structure-property relationships, allow envisioning many promising applications. Consequently, in the past 3 years, application-oriented papers based on discrete synthetic macromolecules emerged. Hence, material science applications such as macromolecular data storage and encryption, self-assembly of discrete structures and foldamers have been the object of many fascinating studies. Moreover, in the area of life sciences, such structures have also been the focus of numerous research studies. Here, it is aimed to highlight these recent applications and to give the reader a critical overview of the future trends in this area of research.
Keywords: data storage; discrete macromolecules; sequence defined polymers; structure–property relationships.
© 2021 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

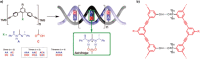

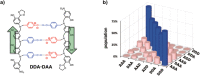
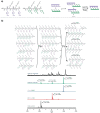

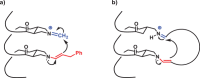
References
-
- Seymour R. B., J. Chem. Educ. 1988, 65, 327.
-
- Feldman D., Des. Monomers Polym. 2008, 11, 1.
-
- Aksakal R., Resmini M., Becer C. R., Polym. Chem. 2016, 7, 171
-
- Aksakal S., Beyer V. P., Aksakal R., Becer C. R., Polym. Chem. 2019, 10, 6622.
-
- Lutz J.‐F., Ouchi M., Liu D. R., Sawamoto M., Science 2013, 341, 1238149. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
