Anti-Tumoral and Anti-Angiogenic Effects of Low-Diluted Phenacetinum on Melanoma
- PMID: 33747916
- PMCID: PMC7966719
- DOI: 10.3389/fonc.2021.597503
Anti-Tumoral and Anti-Angiogenic Effects of Low-Diluted Phenacetinum on Melanoma
Abstract
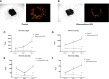
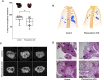
Melanoma is the most aggressive form of skin cancer and the most rapidly expanding cancer in terms of worldwide incidence. If primary cutaneous melanoma is mostly treated with a curative wide local excision, malignant melanoma has a poor prognosis and needs other therapeutic approaches. Angiogenesis is a normal physiological process essential in growth and development, but it also plays a crucial role in crossing from benign to advanced state in cancer. In melanoma progression, angiogenesis is widely involved during the vertical growth phase. Currently, no anti-angiogenic agents are efficient on their own, and combination of treatments will probably be the key to success. In the past, phenacetin was used as an analgesic to relieve pain, causing side effects at large dose and tumor-inducing in humans and animals. By contrast, Phenacetinum low-dilution is often used in skin febrile exanthema, patches profusely scattered on limbs, headache, or flushed face without side effects. Herein are described the in vitro, in vivo, and ex vivo anti-angiogenic and anti-tumoral potentials of Phenacetinum low-dilution in a B16F1 tumor model and endothelial cells. We demonstrate that low-diluted Phenacetinum inhibits in vivo tumor growth and tumor vascularization and thus increases the survival time of B16F1 melanoma induced-C57BL/6 mice. Moreover, Phenacetinum modulates the lung metastasis in a B16F10 induced model. Ex vivo and in vitro, we evidence that low-diluted Phenacetinum inhibits the migration and the recruitment of endothelial cells and leads to an imbalance in the pro-tumoral macrophages and to a structural malformation of the vascular network. All together these results demonstrate highly hopeful anti-tumoral, anti-metastatic, and anti-angiogenic effects of Phenacetinum low-dilution on melanoma. Continued studies are needed to preclinically validate Phenacetinum low-dilution as a complementary or therapeutic strategy for melanoma treatment.
Keywords: angiogenesis; cancer; homeopathy; in vivo; melanoma; metastasis; phenacetin; tumor-associated macrophages.
Copyright © 2021 Fuselier, Quemener, Dufay, Bour, Boulagnon-Rombi, Bouland, Djermoune, Devy, Martiny and Schneider.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

