Case Report: Enfortumab Vedotin for Metastatic Urothelial Carcinoma: A Case Series on the Clinical and Histopathologic Spectrum of Adverse Cutaneous Reactions From Fatal Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis to Dermal Hypersensitivity Reaction
- PMID: 33747934
- PMCID: PMC7970171
- DOI: 10.3389/fonc.2021.621591
Case Report: Enfortumab Vedotin for Metastatic Urothelial Carcinoma: A Case Series on the Clinical and Histopathologic Spectrum of Adverse Cutaneous Reactions From Fatal Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis to Dermal Hypersensitivity Reaction
Abstract
Enfortumab vedotin is a Nectin-4 directed antibody-drug conjugate approved in metastatic urothelial carcinoma following progression on a platinum-containing chemotherapy and immune checkpoint blockade. On-target dermatologic toxicity may occur from Nectin-4 expression in the skin. We highlight a case of Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis following enfortumab infusions that was ultimately fatal. The second case describes an erythema multiforme-like rash with interface dermatitis related to enfortumab. Dermatologic findings, immunohistochemistry studies, and immune profiling are detailed. These cases demonstrate the potentially catastrophic outcomes in some patients treated with enfortumab. Patients must be monitored for cutaneous toxicities with early involvement of dermatology and dermatopathology.
Keywords: SJS/TEN; adverse (side) effects; bladder cancer; enfortumab vedotin; erythema multiform; urothelial cancer.
Copyright © 2021 Viscuse, Marques-Piubelli, Heberton, Parra, Shah, Siefker-Radtke, Gao, Goswami, Ivan, Curry and Campbell.
Conflict of interest statement
MC: Advisory Board/Honorarium: Seattle Genetics, Astellas, Eisai, AstraZeneca, Exelixis, EMD Serono, Pfizer Education grants: Roche, Bristol Myers Squibb, Pfizer Research grants: AstraZeneca, Exelixis, Janssen, Pfizer, EMD Serono. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

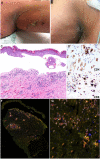
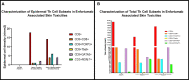

References
-
- Seattle Genetics and Astellas Announce PADCEV® (enfortumab vedotin-ejfv) Significantly Improved Overall Survival in Phase 3 Trial in Previously Treated Locally Advanced or Metastatic Urothelial Cancer [news release]. Bothell, Washington and Tokyo. Available at: https://www.businesswire.com/news/home/20200918005101/en/Seattle-Genetic... (Accessed September 18, 2020). Published September 18, 2020.
-
- Wu S, Adamson AS. Cutaneous toxicity associated with enfortumab vedotin treatment of metastatic urothelial carcinoma. Dermatol Online J (2019) 25(2):13030. - PubMed
-
- Bellmunt J, von der Maase H, Mead GM, Skoneczna I, De Santis M, Daugaard G, et al. . Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC Intergroup Study 30987. J Clin Oncol (2012) 30(10):1107–13. 10.1200/JCO.2011.38.6979 - DOI - PMC - PubMed
-
- von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, et al. . Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol (2000) 18(17):3068–77. 10.1200/JCO.2000.18.17.3068 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

