Radiopharmaceutical Validation for Clinical Use
- PMID: 33747951
- PMCID: PMC7966985
- DOI: 10.3389/fonc.2021.630827
Radiopharmaceutical Validation for Clinical Use
Abstract
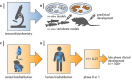
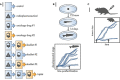
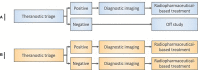
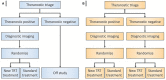
Radiopharmaceuticals are reemerging as attractive anticancer agents, but there are no universally adopted guidelines or standardized procedures for evaluating agent validity before early-phase trial implementation. To validate a radiopharmaceutical, it is desirous for the radiopharmaceutical to be specific, selective, and deliverable against tumors of a given, molecularly defined cancer for which it is intended to treat. In this article, we discuss four levels of evidence-target antigen immunohistochemistry, in vitro and in vivo preclinical experiments, animal biodistribution and dosimetry studies, and first-in-human microdose biodistribution studies-that might be used to justify oncology therapeutic radiopharmaceuticals in a drug-development sequence involving early-phase trials. We discuss common practices for validating radiopharmaceuticals for clinical use, everyday pitfalls, and commonplace operationalizing steps for radiopharmaceutical early-phase trials. We anticipate in the near-term that radiopharmaceutical trials will become a larger proportion of the National Cancer Institute Cancer Therapy Evaluation Program (CTEP) portfolio.
Keywords: nuclear medicine; preclinical; radiation oncology; radiopharmaceutical; validation.
Copyright © 2021 Kunos, Howells, Chauhan, Myint, Bernard, El Khouli and Capala.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Food & Drug Administration . Bioanalytical method validation guidance for industry (2018). U.S. Department of Health and Human Services, Food & Drug Administration, Center for Drug Evaluation and Research (CDER. Available at: https://www.fda.gov/media/70858/download (Accessed August 23, 2020).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

