A Natural Xenogeneic Endometrial Extracellular Matrix Hydrogel Toward Improving Current Human in vitro Models and Future in vivo Applications
- PMID: 33748086
- PMCID: PMC7973233
- DOI: 10.3389/fbioe.2021.639688
A Natural Xenogeneic Endometrial Extracellular Matrix Hydrogel Toward Improving Current Human in vitro Models and Future in vivo Applications
Abstract
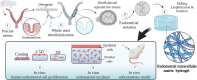
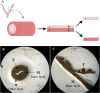
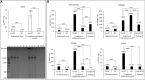
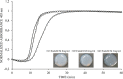
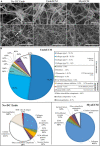
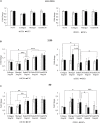
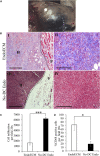
Decellularization techniques support the creation of biocompatible extracellular matrix hydrogels, providing tissue-specific environments for both in vitro cell culture and in vivo tissue regeneration. We obtained endometrium derived from porcine decellularized uteri to create endometrial extracellular matrix (EndoECM) hydrogels. After decellularization and detergent removal, we investigated the physicochemical features of the EndoECM, including gelation kinetics, ultrastructure, and proteomic profile. The matrisome showed conservation of structural and tissue-specific components with low amounts of immunoreactive molecules. EndoECM supported in vitro culture of human endometrial cells in two- and three-dimensional conditions and improved proliferation of endometrial stem cells with respect to collagen and Matrigel. Further, we developed a three-dimensional endometrium-like co-culture system of epithelial and stromal cells from different origins. Endometrial co-cultures remained viable and showed significant remodeling. Finally, EndoECM was injected subcutaneously in immunocompetent mice in a preliminary study to test a possible hypoimmunogenic reaction. Biomimetic endometrial milieus offer new strategies in reproductive techniques and endometrial repair and our findings demonstrate that EndoECM has potential for in vitro endometrial culture and as treatment for endometrial pathologies.
Keywords: 3D culture; decellularization; endometrium; extracellular matrix hydrogels; tissue engineering.
Copyright © 2021 López-Martínez, Campo, de Miguel-Gómez, Faus, Navarro, Díaz, Pellicer, Ferrero and Cervelló.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources

