Three-Dimensional Quantification of Collagen Microstructure During Tensile Mechanical Loading of Skin
- PMID: 33748088
- PMCID: PMC7966723
- DOI: 10.3389/fbioe.2021.642866
Three-Dimensional Quantification of Collagen Microstructure During Tensile Mechanical Loading of Skin
Abstract
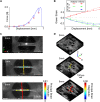
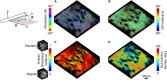
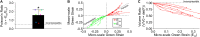
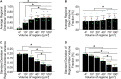
Skin is a heterogeneous tissue that can undergo substantial structural and functional changes with age, disease, or following injury. Understanding how these changes impact the mechanical properties of skin requires three-dimensional (3D) quantification of the tissue microstructure and its kinematics. The goal of this study was to quantify these structure-function relationships via second harmonic generation (SHG) microscopy of mouse skin under tensile mechanical loading. Tissue deformation at the macro- and micro-scale was quantified, and a substantial decrease in tissue volume and a large Poisson's ratio was detected with stretch, indicating the skin differs substantially from the hyperelastic material models historically used to explain its behavior. Additionally, the relative amount of measured strain did not significantly change between length scales, suggesting that the collagen fiber network is uniformly distributing applied strains. Analysis of undeformed collagen fiber organization and volume fraction revealed a length scale dependency for both metrics. 3D analysis of SHG volumes also showed that collagen fiber alignment increased in the direction of stretch, but fiber volume fraction did not change. Interestingly, 3D fiber kinematics was found to have a non-affine relationship with tissue deformation, and an affine transformation of the micro-scale fiber network overestimates the amount of fiber realignment. This result, along with the other outcomes, highlights the importance of accurate, scale-matched 3D experimental measurements when developing multi-scale models of skin mechanical function.
Keywords: collagen; kinematics; microstructure; multiscale; second harmonic generation; skin; structure-function relationship.
Copyright © 2021 Woessner, Jones, Witt, Sander and Quinn.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

