Prostate-Specific Membrane Antigen (PSMA) Promotes Angiogenesis of Glioblastoma Through Interacting With ITGB4 and Regulating NF-κB Signaling Pathway
- PMID: 33748101
- PMCID: PMC7969793
- DOI: 10.3389/fcell.2021.598377
Prostate-Specific Membrane Antigen (PSMA) Promotes Angiogenesis of Glioblastoma Through Interacting With ITGB4 and Regulating NF-κB Signaling Pathway
Abstract
Background: Glioblastoma multiforme (GBM) is the most common primary malignant tumor in the central nervous system (CNS), causing the extremely poor prognosis. Combining the role of angiogenesis in tumor progression and the role of prostate-specific membrane antigen (PSMA) in angiogenesis, this study aims to explore the functions of PSMA in GBM.
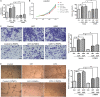
Methods: Clinical GBM specimens were collected from 60 patients who accepted surgical treatment in Fudan University Shanghai Cancer Center between January 2018 and June 2019. Immunohistochemical staining was used to detect PSMA and CD31 expression in GBM tissues. Prognostic significance of PSMA was evaluated by bioinformatics. Human umbilical vein endothelial cells (HUVECs) transfected with PSMA overexpression plasmids or cultured with conditioned medium collected based on GBM cells, were used for CCK8, Transwell and tube formation assays. High-throughput sequencing and immunoprecipitation were used to explore the underlying mechanism. Furthermore, the in vivo experiment had been also conducted.
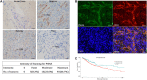
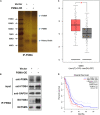
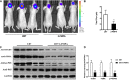
Results: We demonstrated that PSMA was abundantly expressed in endothelium of vessels of GBM tissues but not in vessels of normal tissues, which was significantly correlated with poor prognosis. Overexpression of PSMA could promotes proliferation, invasion and tube formation ability of human umbilical vein endothelial cells (HUVECs). Moreover, U87 or U251 conditioned medium could upregulated PSMA expression and induce similar effects on phenotypes of HUVECs, all of which could be partially attenuated by 2-PMPA treatment. The mechanistic study revealed that PSMA might promote angiogenesis of GBM through interacting with Integrin β4 (ITGB4) and activating NF-κB signaling pathway. The in vivo growth of GBM could be alleviated by the treatment of 2-PMPA.
Conclusion: This study identified PSMA as a critical regulator in angiogenesis and progression of GBM, which might be a promising therapeutic target for GBM treatment.
Keywords: GBM; HUVEC; PSMA; angiogenesis; tumor progression.
Copyright © 2021 Gao, Zheng, Li, Feng, Chen, Hao, Lv, Zhou and Cao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Secreted phosphoprotein 1 promotes angiogenesis of glioblastoma through upregulating PSMA expression via transcription factor HIF1α.Acta Biochim Biophys Sin (Shanghai). 2022 Oct 25;55(3):417-425. doi: 10.3724/abbs.2022157. Acta Biochim Biophys Sin (Shanghai). 2022. PMID: 36305723 Free PMC article.
-
ALKBH5 is a prognostic factor and promotes the angiogenesis of glioblastoma.Sci Rep. 2024 Jan 14;14(1):1303. doi: 10.1038/s41598-024-51994-9. Sci Rep. 2024. PMID: 38221546 Free PMC article.
-
Prostate-specific membrane antigen as a potential novel vascular target for treatment of glioblastoma multiforme.Arch Pathol Lab Med. 2011 Nov;135(11):1486-9. doi: 10.5858/arpa.2010-0740-OA. Arch Pathol Lab Med. 2011. PMID: 22032578
-
Natural products as promising targets in glioblastoma multiforme: a focus on NF-κB signaling pathway.Pharmacol Rep. 2020 Apr;72(2):285-295. doi: 10.1007/s43440-020-00081-7. Epub 2020 Mar 9. Pharmacol Rep. 2020. PMID: 32152926
-
The autotaxin-lysophosphatidic acid-lysophosphatidic acid receptor cascade: proposal of a novel potential therapeutic target for treating glioblastoma multiforme.Lipids Health Dis. 2015 Jun 18;14:56. doi: 10.1186/s12944-015-0059-5. Lipids Health Dis. 2015. PMID: 26084470 Free PMC article. Review.
Cited by
-
Advances in Molecular Regulation of Prostate Cancer Cells by Top Natural Products of Malaysia.Curr Issues Mol Biol. 2023 Feb 9;45(2):1536-1567. doi: 10.3390/cimb45020099. Curr Issues Mol Biol. 2023. PMID: 36826044 Free PMC article. Review.
-
Application of 18F Prostate-Specific Membrane Antigen Positron Emission Tomography/Computed Tomography in Monitoring Gastric Metastasis and Cancer Thrombi from Renal Cell Carcinoma.J Oncol. 2022 Feb 4;2022:5681463. doi: 10.1155/2022/5681463. eCollection 2022. J Oncol. 2022. PMID: 35154318 Free PMC article.
-
Phosphonates and Phosphonate Prodrugs in Medicinal Chemistry: Past Successes and Future Prospects.Front Chem. 2022 May 20;10:889737. doi: 10.3389/fchem.2022.889737. eCollection 2022. Front Chem. 2022. PMID: 35668826 Free PMC article. Review.
-
[68Ga]Ga-PSMA PET/MRI, histological PSMA expression and preliminary experience with [177Lu]Lu-PSMA therapy in relapsing high-grade glioma.Front Oncol. 2022 Sep 2;12:980058. doi: 10.3389/fonc.2022.980058. eCollection 2022. Front Oncol. 2022. PMID: 36119502 Free PMC article.
-
The clinical application of 68Ga-PSMA PET/CT and regulating mechanism of PSMA expression in patients with brain metastases of lung cancer.Transl Oncol. 2023 Feb;28:101616. doi: 10.1016/j.tranon.2023.101616. Epub 2023 Jan 6. Transl Oncol. 2023. PMID: 36621073 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

