Role of Lipid Metabolism and Signaling in Mammalian Oocyte Maturation, Quality, and Acquisition of Competence
- PMID: 33748128
- PMCID: PMC7973101
- DOI: 10.3389/fcell.2021.639704
Role of Lipid Metabolism and Signaling in Mammalian Oocyte Maturation, Quality, and Acquisition of Competence
Abstract
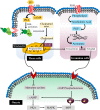
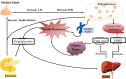
It has been found that the quality of oocytes from obese women has been compromised and subsequent embryos displayed arrested development. The compromised quality may be either due to the poor or rich metabolic conditions such as imbalance or excession of lipids during oocyte development. Generally, lipids are mainly stored in the form of lipid droplets and are an important source of energy metabolism. Similarly, lipids are also essential signaling molecules involved in various biological cascades of oocyte maturation, growth and oocyte competence acquisition. To understand the role of lipids in controlling the oocyte development, we have comprehensively and concisely reviewed the literature and described the role of lipid metabolism in oocyte quality and maturation. Moreover, we have also presented a simplified model of fatty acid metabolism along with its implication on determining the oocyte quality and cryopreservation for fertilization.
Keywords: fertilization; lipid metabolism; obesity; oocyte development; oocyte maturation.
Copyright © 2021 Khan, Jiang, Hameed and Shi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Apparicio M., Ferreira C. R., Tata A., Santos V. G., Alves A. E., Mostachio G. Q., et al. (2012). Chemical composition of lipids present in cat and dog oocyte by matrix-assisted desorption ionization mass spectrometry (MALDI- MS). Reprod. Domest. Anim. 47(Suppl. 6) 113–117. 10.1111/rda.12003 - DOI - PubMed
-
- Auclair S., Uzbekov R., Elis S., Sanchez L., Kireev I., Lardic L., et al. (2013). Absence of cumulus cells during in vitro maturation affects lipid metabolism in bovine oocytes. Am. J. Physiol. Endocrinol. Metab. 304 E599–E613. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

