SARAF and Orai1 Contribute to Endothelial Cell Activation and Angiogenesis
- PMID: 33748129
- PMCID: PMC7970240
- DOI: 10.3389/fcell.2021.639952
SARAF and Orai1 Contribute to Endothelial Cell Activation and Angiogenesis
Erratum in
-
Corrigendum: SARAF and Orai1 Contribute to Endothelial Cell Activation and Angiogenesis.Front Cell Dev Biol. 2021 Apr 20;9:683097. doi: 10.3389/fcell.2021.683097. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33959619 Free PMC article.
Abstract
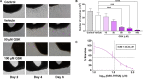
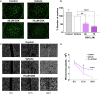
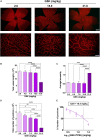
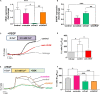
Angiogenesis is a multistep process that controls endothelial cells (ECs) functioning to form new blood vessels from preexisting vascular beds. This process is tightly regulated by pro-angiogenic factors, such as vascular endothelial growth factor (VEGF), which promote signaling pathways involving the increase in the intracellular Ca2+ concentration ([Ca2+]i). Recent evidence suggests that store-operated calcium entry (SOCE) might play a role in angiogenesis. However, little is known regarding the role of SARAF, SOCE-associated regulatory factor, and Orai1, the pore-forming subunit of the store-operated calcium channel (SOCC), in angiogenesis. Here, we show that SOCE inhibition with GSK-7975A blocks aorta sprouting, as well as human umbilical vein endothelial cell (HUVEC) tube formation and migration. The intraperitoneal injection of GSK-7975A also delays the development of retinal vasculature assessed at postnatal day 6 in mice, since it reduces vessel length and the number of junctions, while it increases lacunarity. Moreover, we find that SARAF and Orai1 are involved in VEGF-mediated [Ca2+]i increase, and their knockdown using siRNA impairs HUVEC tube formation, proliferation, and migration. Finally, immunostaining and in situ proximity ligation assays indicate that SARAF likely interacts with Orai1 in HUVECs. Therefore, these findings show for the first time a functional interaction between SARAF and Orai1 in ECs and highlight their essential role in different steps of the angiogenesis process.
Keywords: HUVEC; Orai1; SARAF; SOCE; angiogenesis.
Copyright © 2021 Galeano-Otero, Del Toro, Khatib, Rosado, Ordóñez-Fernández and Smani.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Adair T. H., Montani J. P. (2010). Overview of Angiogenesis. San Rafael, CA: Morgan & Claypool Life Sciences. - PubMed
-
- Albarran L., Lopez J. J., Woodard G. E., Salido G. M., Rosado J. A. (2016b). Store-operated Ca2+ entry-associated regulatory factor (SARAF) plays an important role in the regulation of arachidonate-regulated Ca2+ (ARC) channels. J. Biol. Chem. 291 6982–6988. 10.1074/jbc.M115.704940 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

