Somatic and de novo Germline Variants of MEDs in Human Neural Tube Defects
- PMID: 33748132
- PMCID: PMC7969791
- DOI: 10.3389/fcell.2021.641831
Somatic and de novo Germline Variants of MEDs in Human Neural Tube Defects
Abstract
Background: Neural tube defects (NTDs) are among the most common and severe congenital defects in humans. Their genetic etiology is complex and remains poorly understood. The Mediator complex (MED) plays a vital role in neural tube development in animal models. However, no studies have yet examined the role of its human homolog in the etiology of NTDs.
Methods: In this study, 48 pairs of neural lesion site and umbilical cord tissues from NTD and 21 case-parent trios were involved in screening for NTD-related somatic and germline de novo variants. A series of functional cell assays were performed. We generated a Med12 p.Arg1784Cys knock-in mouse using CRISPR/Cas9 technology to validate the human findings.
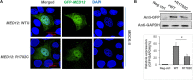
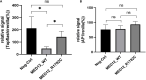
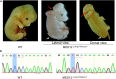
Results: One somatic variant, MED12 p.Arg1782Cys, was identified in the lesion site tissue from an NTD fetus. This variant was absent in any other normal tissue from different germ layers of the same case. In 21 case-parent trios, one de novo stop-gain variant, MED13L p.Arg1760∗, was identified. Cellular functional studies showed that MED12 p.Arg1782Cys decreased MED12 protein level and affected the regulation of MED12 on the canonical-WNT signaling pathway. The Med12 p.Arg1784Cys knock-in mouse exhibited exencephaly and spina bifida.
Conclusion: These findings provide strong evidence that functional variants of MED genes are associated with the etiology of some NTDs. We demonstrated a potentially important role for somatic variants in the occurrence of NTDs. Our study is the first study in which an NTD-related variant identified in humans was validated in mice using CRISPR/Cas9 technology.
Keywords: CRISPR/Cas9; MEDs; de novo variant; neural tube defects; somatic variants.
Copyright © 2021 Tian, Cao, Chen, Jin, Li, Han, Lin, Wlodarczyk, Finnell, Yuan, Wang, Ren and Lei.
Conflict of interest statement
RF and BW formerly consulted with the now dissolved TeratOmic Consulting LLC. RF also receives travel funds to attend editorial board meetings of the Journal of Reproductive and Developmental Medicine published out of the Red Hospital of Fudan University. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Somatic mutations in planar cell polarity genes in neural tissue from human fetuses with neural tube defects.Hum Genet. 2020 Oct;139(10):1299-1314. doi: 10.1007/s00439-020-02172-0. Epub 2020 Apr 30. Hum Genet. 2020. PMID: 32356230 Free PMC article.
-
Genetic analysis of Wnt/PCP genes in neural tube defects.BMC Med Genomics. 2018 Apr 4;11(1):38. doi: 10.1186/s12920-018-0355-9. BMC Med Genomics. 2018. PMID: 29618362 Free PMC article.
-
Interaction between Maternal and Paternal SHMT1 C1420T Predisposes to Neural Tube Defects in the Fetus: Evidence from Case-Control and Family-Based Triad Approaches.Birth Defects Res. 2017 Jul 17;109(13):1020-1029. doi: 10.1002/bdr2.23623. Birth Defects Res. 2017. PMID: 28762673
-
Mini-review: toward understanding mechanisms of genetic neural tube defects in mice.Teratology. 1999 Nov;60(5):292-305. doi: 10.1002/(SICI)1096-9926(199911)60:5<292::AID-TERA10>3.0.CO;2-6. Teratology. 1999. PMID: 10525207 Review.
-
Spina bifida and other neural tube defects.Curr Probl Pediatr. 2000 Nov-Dec;30(10):313-32. doi: 10.1067/mpp.2000.112052. Curr Probl Pediatr. 2000. PMID: 11147289 Review.
Cited by
-
Novel insights into the phenotypic spectrum and pathogenesis of Hardikar syndrome.Genet Med. 2024 Oct;26(10):101222. doi: 10.1016/j.gim.2024.101222. Epub 2024 Jul 20. Genet Med. 2024. PMID: 39045790
-
MED13L-related intellectual disability due to paternal germinal mosaicism.Cold Spring Harb Mol Case Stud. 2022 Jan 10;8(1):a006124. doi: 10.1101/mcs.a006124. Print 2022 Jan. Cold Spring Harb Mol Case Stud. 2022. PMID: 34654706 Free PMC article.
-
Pathogenesis of neural tube defects: The regulation and disruption of cellular processes underlying neural tube closure.WIREs Mech Dis. 2022 Sep;14(5):e1559. doi: 10.1002/wsbm.1559. Epub 2022 May 3. WIREs Mech Dis. 2022. PMID: 35504597 Free PMC article. Review.
-
Unraveling the complex genetics of neural tube defects: From biological models to human genomics and back.Genesis. 2021 Nov;59(11):e23459. doi: 10.1002/dvg.23459. Epub 2021 Oct 29. Genesis. 2021. PMID: 34713546 Free PMC article. Review.
-
Spina Bifida: A Review of the Genetics, Pathophysiology and Emerging Cellular Therapies.J Dev Biol. 2022 Jun 6;10(2):22. doi: 10.3390/jdb10020022. J Dev Biol. 2022. PMID: 35735913 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

