Mechanism and Control of Meiotic DNA Double-Strand Break Formation in S. cerevisiae
- PMID: 33748134
- PMCID: PMC7968521
- DOI: 10.3389/fcell.2021.642737
Mechanism and Control of Meiotic DNA Double-Strand Break Formation in S. cerevisiae
Abstract
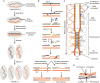
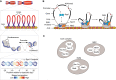
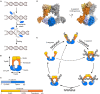
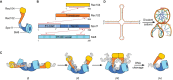
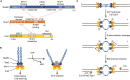
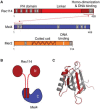
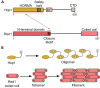
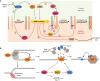
Developmentally programmed formation of DNA double-strand breaks (DSBs) by Spo11 initiates a recombination mechanism that promotes synapsis and the subsequent segregation of homologous chromosomes during meiosis. Although DSBs are induced to high levels in meiosis, their formation and repair are tightly regulated to minimize potentially dangerous consequences for genomic integrity. In S. cerevisiae, nine proteins participate with Spo11 in DSB formation, but their molecular functions have been challenging to define. Here, we describe our current view of the mechanism of meiotic DSB formation based on recent advances in the characterization of the structure and function of DSB proteins and discuss regulatory pathways in the light of recent models.
Keywords: DNA recombination; Spo11; double-strand break; meiosis; phase separation.
Copyright © 2021 Yadav and Claeys Bouuaert.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

