Leveraging Oral Drug Development to a Next Level: Impact of the IMI-Funded OrBiTo Project on Patient Healthcare
- PMID: 33748152
- PMCID: PMC7973356
- DOI: 10.3389/fmed.2021.480706
Leveraging Oral Drug Development to a Next Level: Impact of the IMI-Funded OrBiTo Project on Patient Healthcare
Abstract
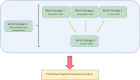
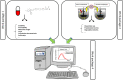
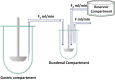
A thorough understanding of the behavior of drug formulations in the human gastrointestinal (GI) tract is essential when working in the field of oral drug development in a pharmaceutical company. For orally administered drug products, various GI processes, including disintegration of the drug formulation, drugrelease, dissolution, precipitation, degradation, dosage form transit and permeation, dictate absorption into the systemic circulation. These processes are not always fully captured in predictive in vitro and in silico tools, as commonly applied in the pre-clinical stage of formulation drug development. A collaborative initiative focused on the science of oral biopharmaceutics was established in 2012 between academic institutions and industrial companies to innovate, optimize and validate these in vitro and in silico biopharmaceutical tools. From that perspective, the predictive power of these models can be revised and, if necessary, optimized to improve the accuracy toward predictions of the in vivo performance of orally administered drug products in patients. The IMI/EFPIA-funded "Oral Bioavailability Tools (OrBiTo)" project aimed to improve our fundamental understanding of the GI absorption process. The gathered information was integrated into the development of new (or already existing) laboratory tests and computer-based methods in order to deliver more accurate predictions of drug product behavior in a real-life setting. These methods were validated with the use of industrial data. Crucially, the ultimate goal of the project was to set up a scientific framework (i.e., decision trees) to guide the use of these new tools in drug development. The project aimed to facilitate and accelerate the formulation development process and to significantly reduce the need for animal experiments in this area as well as for human clinical studies in the future. With respect to the positive outcome for patients, high-quality oral medicines will be developed where the required dose is well-calculated and consistently provides an optimal clinical effect. In a first step, this manuscript summarizes the setup of the project and how data were collected across the different work packages. In a second step, case studies of how this project contributed to improved knowledge of oral drug delivery which can be used to develop improved products for patients will be illustrated.
Keywords: EFPIA; IMI; oral absorption; oral biopharmaceutical tools; oral formulations; patient health care; pharmacokinetic.
Copyright © 2021 Hens, Augustijns, Lennernäs, McAllister and Abrahamsson.
Conflict of interest statement
BH and MM are employed by Pfizer UK. BA is employed by AstraZeneca. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

