Performance of a Point of Care Test for Detecting IgM and IgG Antibodies Against SARS-CoV-2 and Seroprevalence in Blood Donors and Health Care Workers in Panama
- PMID: 33748157
- PMCID: PMC7968482
- DOI: 10.3389/fmed.2021.616106
Performance of a Point of Care Test for Detecting IgM and IgG Antibodies Against SARS-CoV-2 and Seroprevalence in Blood Donors and Health Care Workers in Panama
Abstract
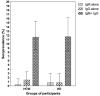
Novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the etiologic agent of the ongoing coronavirus disease 2019 (COVID-19) pandemic, which has reached 28 million cases worldwide in 1 year. The serological detection of antibodies against the virus will play a pivotal role in complementing molecular tests to improve diagnostic accuracy, contact tracing, vaccine efficacy testing, and seroprevalence surveillance. Here, we aimed first to evaluate a lateral flow assay's ability to identify specific IgM and IgG antibodies against SARS-CoV-2 and second, to report the seroprevalence estimates of these antibodies among health care workers and healthy volunteer blood donors in Panama. We recruited study participants between April 30th and July 7th, 2020. For the test validation and performance evaluation, we analyzed serum samples from participants with clinical symptoms and confirmed positive RT-PCR for SARS-CoV-2, and a set of pre-pandemic serum samples. We used two by two table analysis to determine the test positive and negative percentage agreement as well as the Kappa agreement value with a 95% confidence interval. Then, we used the lateral flow assay to determine seroprevalence among serum samples from COVID-19 patients, potentially exposed health care workers, and healthy volunteer donors. Our results show this assay reached a positive percent agreement of 97.2% (95% CI 84.2-100.0%) for detecting both IgM and IgG. The assay showed a Kappa of 0.898 (95%CI 0.811-0.985) and 0.918 (95% CI 0.839-0.997) for IgM and IgG, respectively. The evaluation of serum samples from hospitalized COVID-19 patients indicates a correlation between test sensitivity and the number of days since symptom onset; the highest positive percent agreement [87% (95% CI 67.0-96.3%)] was observed at ≥15 days post-symptom onset (PSO). We found an overall antibody seroprevalence of 11.6% (95% CI 8.5-15.8%) among both health care workers and healthy blood donors. Our findings suggest this lateral flow assay could contribute significantly to implementing seroprevalence testing in locations with active community transmission of SARS-CoV-2.
Keywords: COVID-19; Panama; biomarker; diagnosis; immunochromatographic assay; serology.
Copyright © 2021 Villarreal, Rangel, Zhang, Wong, Britton, Fernandez, Pérez, Oviedo, Restrepo, Carreirra, Sambrano, Eskildsen, De La Guardia, Flores-Cuadra, Carrera, Zaldivar, Franco, López-Vergès, Zhang, Fan, Wang, Sáez-Llorens, DeAntonio, Torres-Atencio, Blanco, Subía, Mudarra, Benzadon, Valverde, López, Hurtado, Rivas, Jurado, Carvallo, Rodriguez, Perez, Morris, Luque, Cortez, Ortega-Barria, Kosagisharaf, Lleonart, Li and Goodridge.
Conflict of interest statement
EO-B was employed by the GlaxoSmithKline. CL, XZ, DZ, and FF were employed by company Beijing Zhongke Jianlan Biotechnology Co., Ltd., and BW was employed by company Beijing Kewei Clinical Diagnostic Reagent Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- WHO Coronavirus Disease (COVID-19) Dashboard. Available online at: https://covid19.who.int/?gclid=CjwKCAjw4_H6BRALEiwAvgfzq34TfRvRSv7OVn2CV... (accessed January 12, 2021).
-
- Organization PAH . COVID-19 - PAHO/WHO Response, Report 20. In: Book COVID-19 - PAHO/WHO Response, Report 20. Panama city: Pan American Health Organization; (2020).
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

