CRISPR/Cas: Advances, Limitations, and Applications for Precision Cancer Research
- PMID: 33748164
- PMCID: PMC7965951
- DOI: 10.3389/fmed.2021.649896
CRISPR/Cas: Advances, Limitations, and Applications for Precision Cancer Research
Abstract
Cancer is one of the most leading causes of mortalities worldwide. It is caused by the accumulation of genetic and epigenetic alterations in 2 types of genes: tumor suppressor genes (TSGs) and proto-oncogenes. In recent years, development of the clustered regularly interspaced short palindromic repeats (CRISPR) technology has revolutionized genome engineering for different cancer research ranging for research ranging from fundamental science to translational medicine and precise cancer treatment. The CRISPR/CRISPR associated proteins (CRISPR/Cas) are prokaryote-derived genome editing systems that have enabled researchers to detect, image, manipulate and annotate specific DNA and RNA sequences in various types of living cells. The CRISPR/Cas systems have significant contributions to discovery of proto-oncogenes and TSGs, tumor cell epigenome normalization, targeted delivery, identification of drug resistance mechanisms, development of high-throughput genetic screening, tumor models establishment, and cancer immunotherapy and gene therapy in clinics. Robust technical improvements in CRISPR/Cas systems have shown a considerable degree of efficacy, specificity, and flexibility to target the specific locus in the genome for the desired applications. Recent developments in CRISPRs technology offers a significant hope of medical cure against cancer and other deadly diseases. Despite significant improvements in this field, several technical challenges need to be addressed, such as off-target activity, insufficient indel or low homology-directed repair (HDR) efficiency, in vivo delivery of the Cas system components, and immune responses. This study aims to overview the recent technological advancements, preclinical and perspectives on clinical applications of CRISPR along with their advantages and limitations. Moreover, the potential applications of CRISPR/Cas in precise cancer tumor research, genetic, and other precise cancer treatments discussed.
Keywords: CRiSPR/Cas; cancer; clustered regularly interspaced short palindromic repeats; diagnosis; genetic editing; precise cancer treatment; precision medicine.
Copyright © 2021 Yang, Xu, Ge and Lai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
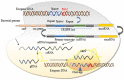
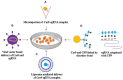
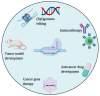
References
-
- Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, Abdel-Rahman O, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-Adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol. (2019) 5:1749–68. 10.1001/jamaoncol.2019.2996 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

