Endothelial Lipase Is Involved in Cold-Induced High-Density Lipoprotein Turnover and Reverse Cholesterol Transport in Mice
- PMID: 33748195
- PMCID: PMC7973023
- DOI: 10.3389/fcvm.2021.628235
Endothelial Lipase Is Involved in Cold-Induced High-Density Lipoprotein Turnover and Reverse Cholesterol Transport in Mice
Abstract
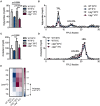
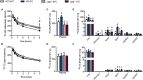
The physiologic activation of thermogenic brown and white adipose tissues (BAT/WAT) by cold exposure triggers heat production by adaptive thermogenesis, a process known to ameliorate hyperlipidemia and protect from atherosclerosis. Mechanistically, it has been shown that thermogenic activation increases lipoprotein lipase (LPL)-dependent hydrolysis of triglyceride-rich lipoproteins (TRL) and accelerates the generation of cholesterol-enriched remnants and high-density lipoprotein (HDL), which promotes cholesterol flux from the periphery to the liver. HDL is also subjected to hydrolysis by endothelial lipase (EL) (encoded by LIPG). Genome-wide association studies have identified various variants of EL that are associated with altered HDL cholesterol levels. However, a potential role of EL in BAT-mediated HDL metabolism has not been investigated so far. In the present study, we show that in mice, cold-stimulated activation of thermogenic adipocytes induced expression of Lipg in BAT and inguinal WAT but that loss of Lipg did not affect gene expression of thermogenic markers. Furthermore, in both wild type (WT) and Lipg-deficient mice, activation of thermogenesis resulted in a decline of HDL cholesterol levels. However, cold-induced remodeling of the HDL lipid composition was different between WT and Lipg-deficient mice. Notably, radioactive tracer studies with double-labeled HDL indicated that cold-induced hepatic HDL cholesterol clearance was lower in Lipg-deficient mice. Moreover, this reduced clearance was associated with impaired macrophage-to-feces cholesterol transport. Overall, these data indicate that EL is a determinant of HDL lipid composition, cholesterol flux, and HDL turnover in conditions of high thermogenic activity.
Keywords: HDL; brown adipose tissue; cholesterol; endothelial lipase; lipidomics.
Copyright © 2021 Schaltenberg, John, Heine, Haumann, Rinninger, Scheja, Heeren and Worthmann.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Thermogenic adipocytes promote HDL turnover and reverse cholesterol transport.Nat Commun. 2017 Apr 19;8:15010. doi: 10.1038/ncomms15010. Nat Commun. 2017. PMID: 28422089 Free PMC article. Clinical Trial.
-
Lysosomal lipoprotein processing in endothelial cells stimulates adipose tissue thermogenic adaptation.Cell Metab. 2021 Mar 2;33(3):547-564.e7. doi: 10.1016/j.cmet.2020.12.001. Epub 2020 Dec 22. Cell Metab. 2021. PMID: 33357458
-
Role of Endothelial Cell Lipoprotein Lipase for Brown Adipose Tissue Lipid and Glucose Handling.Front Physiol. 2022 Mar 29;13:859671. doi: 10.3389/fphys.2022.859671. eCollection 2022. Front Physiol. 2022. PMID: 35422714 Free PMC article.
-
Role of thermogenic adipose tissue in lipid metabolism and atherosclerotic cardiovascular disease: lessons from studies in mice and humans.Cardiovasc Res. 2023 May 2;119(4):905-918. doi: 10.1093/cvr/cvac131. Cardiovasc Res. 2023. PMID: 35944189 Free PMC article. Review.
-
Brown adipose tissue and lipid metabolism.Curr Opin Lipidol. 2018 Jun;29(3):180-185. doi: 10.1097/MOL.0000000000000504. Curr Opin Lipidol. 2018. PMID: 29718003 Review.
Cited by
-
Inactivation of Type 3 Deiodinase Results in Life-long Changes in the Brown Adipose Tissue Transcriptome in the Male Mouse.Endocrinology. 2022 May 1;163(5):bqac026. doi: 10.1210/endocr/bqac026. Endocrinology. 2022. PMID: 35238380 Free PMC article.
-
Whole Transcriptome Profiling of the Effects of Cadmium on the Liver of the Xiangxi Yellow Heifer.Front Vet Sci. 2022 Apr 14;9:846662. doi: 10.3389/fvets.2022.846662. eCollection 2022. Front Vet Sci. 2022. PMID: 35498726 Free PMC article.
-
Cold-Induced Lipoprotein Clearance in Cyp7b1-Deficient Mice.Front Cell Dev Biol. 2022 Apr 11;10:836741. doi: 10.3389/fcell.2022.836741. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35478959 Free PMC article.
-
Characterization of pig skeletal muscle transcriptomes in response to low temperature.Vet Med Sci. 2023 Jan;9(1):181-190. doi: 10.1002/vms3.1025. Epub 2022 Dec 8. Vet Med Sci. 2023. PMID: 36480456 Free PMC article.
-
Lipid Transport in Brown Adipocyte Thermogenesis.Front Physiol. 2021 Dec 23;12:787535. doi: 10.3389/fphys.2021.787535. eCollection 2021. Front Physiol. 2021. PMID: 35002769 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

