Theoretical Models and Computational Analysis of Action Potential Dispersion for Cardiac Arrhythmia Risk Stratification
- PMID: 33748198
- PMCID: PMC7973016
- DOI: 10.3389/fcvm.2021.649489
Theoretical Models and Computational Analysis of Action Potential Dispersion for Cardiac Arrhythmia Risk Stratification
Abstract
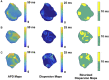
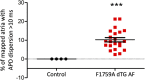
Reentrant cardiac arrhythmias such as atrial fibrillation (AF) and ventricular fibrillation (VF) are common cardiac arrhythmias that account for substantial morbidity and mortality throughout the world. However, the mechanisms and optimal ablation treatment strategies for such arrhythmias are still unclear. Using 2D optical mapping of a mouse model with AF and VF, we have identified regional heterogeneity of the action potential duration (APD) in the atria and ventricles of the heart as key drivers for the initiation and persistence of reentry. The purpose of this paper is to discuss theoretical patterns of dispersion, demonstrate patterns of dispersion seen in our mouse model and discuss the computational analysis of APD dispersion patterns. These analyses and discussions may lead to better understanding of dispersion patterns in patients with these arrhythmias, as well as help comprehend whether and how reducing dispersion can lead to arrhythmia risk stratification and treatment strategies for arrhythmias.
Keywords: action potential dispersion; action potential duration; cardiac arrhythmia; heart; optical mapping of calcium and action potentials.
Copyright © 2021 Avula, Melki, Kushner, Liang and Wan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling editor declared a past collaboration with the author EW.
Figures



Similar articles
-
Heterogeneity of the action potential duration is required for sustained atrial fibrillation.JCI Insight. 2019 Apr 25;5(11):e128765. doi: 10.1172/jci.insight.128765. JCI Insight. 2019. PMID: 31021331 Free PMC article.
-
Mechanisms linking electrical alternans and clinical ventricular arrhythmia in human heart failure.Heart Rhythm. 2016 Sep;13(9):1922-31. doi: 10.1016/j.hrthm.2016.05.017. Epub 2016 May 20. Heart Rhythm. 2016. PMID: 27215536 Free PMC article.
-
Action potential duration gradient protects the right atrium from fibrillating.Conf Proc IEEE Eng Med Biol Soc. 2006;2006:3978-81. doi: 10.1109/IEMBS.2006.260522. Conf Proc IEEE Eng Med Biol Soc. 2006. PMID: 17947064
-
Optical Mapping of Ventricular Fibrillation Dynamics.Adv Exp Med Biol. 2015;859:313-42. doi: 10.1007/978-3-319-17641-3_13. Adv Exp Med Biol. 2015. PMID: 26238059 Review.
-
Mapping of atrial fibrillation: strategies to understand an enigmatic arrhythmia.Herzschrittmacherther Elektrophysiol. 2018 Sep;29(3):307-314. doi: 10.1007/s00399-018-0586-7. Epub 2018 Sep 13. Herzschrittmacherther Elektrophysiol. 2018. PMID: 30215110 Review. English.
Cited by
-
Arrhythmogenic mechanism of a novel ryanodine receptor mutation underlying sudden cardiac death.Europace. 2023 Jul 4;25(7):euad220. doi: 10.1093/europace/euad220. Europace. 2023. PMID: 37466361 Free PMC article.
-
Heterogeneity of ventricular action potentials in neonatal rat cardiomyocytes and methodological aspects of patch clamp measurements.Front Physiol. 2025 Feb 21;16:1537345. doi: 10.3389/fphys.2025.1537345. eCollection 2025. Front Physiol. 2025. PMID: 40061457 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

