Changes in Oviductal Cells and Small Extracellular Vesicles miRNAs in Pregnant Cows
- PMID: 33748215
- PMCID: PMC7969882
- DOI: 10.3389/fvets.2021.639752
Changes in Oviductal Cells and Small Extracellular Vesicles miRNAs in Pregnant Cows
Abstract
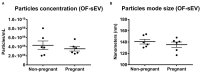
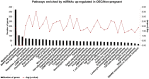
Early embryonic development occurs in the oviduct, where an ideal microenvironment is provided by the epithelial cells and by the oviductal fluid produced by these cells. The oviductal fluid contains small extracellular vesicles (sEVs), which through their contents, including microRNAs (miRNAs), can ensure proper cell communication between the mother and the embryo. However, little is known about the modulation of miRNAs within oviductal epithelial cells (OECs) and sEVs from the oviductal fluid in pregnant cows. In this study, we evaluate the miRNAs profile in sEVs from the oviductal flushing (OF-sEVs) and OECs from pregnant cows compared to non-pregnant, at 120 h after ovulation induction. In OF-sEVs, eight miRNAs (bta-miR-126-5p, bta-miR-129, bta-miR-140, bta-miR-188, bta-miR-219, bta-miR-345-3p, bta-miR-4523, and bta-miR-760-3p) were up-regulated in pregnant and one miRNA (bta-miR-331-5p) was up-regulated in non-pregnant cows. In OECs, six miRNAs (bta-miR-133b, bta-miR-205, bta-miR-584, bta-miR-551a, bta-miR-1193, and bta-miR-1225-3p) were up-regulated in non-pregnant and none was up-regulated in pregnant cows. Our results suggest that embryonic maternal communication mediated by sEVs initiates in the oviduct, and the passage of gametes and the embryo presence modulate miRNAs contents of sEVs and OECs. Furthermore, we demonstrated the transcriptional levels modulation of selected genes in OECs in pregnant cows. Therefore, the embryonic-maternal crosstalk potentially begins during early embryonic development in the oviduct through the modulation of miRNAs in OECs and sEVs in pregnant cows.
Keywords: bovine; embryo maternal-communication; miRNAs; oviductal cells; oviductal fluid; reproduction; small extracellular vesicles.
Copyright © 2021 Mazzarella, Bastos, Bridi, del Collado, Andrade, Pinzon, Prado, Silva, Meirelles, Pugliesi, Perecin and da Silveira.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Extracellular vesicles-coupled miRNAs from oviduct and uterus modulate signaling pathways related to lipid metabolism and bovine early embryo development.J Anim Sci Biotechnol. 2024 Apr 4;15(1):51. doi: 10.1186/s40104-024-01008-5. J Anim Sci Biotechnol. 2024. PMID: 38570884 Free PMC article.
-
Sexual dimorphic miRNA-mediated response of bovine elongated embryos to the maternal microenvironment.PLoS One. 2024 Feb 29;19(2):e0298835. doi: 10.1371/journal.pone.0298835. eCollection 2024. PLoS One. 2024. PMID: 38422042 Free PMC article.
-
Characterization of miRNAs in Milk Small Extracellular Vesicles from Enzootic Bovine Leukosis Cattle.Int J Mol Sci. 2022 Sep 15;23(18):10782. doi: 10.3390/ijms231810782. Int J Mol Sci. 2022. PMID: 36142686 Free PMC article.
-
The role of the oviduct and extracellular vesicles during early embryo development in bovine.Anim Reprod. 2022 Apr 20;19(1):e20220015. doi: 10.1590/1984-3143-AR2022-0015. eCollection 2022. Anim Reprod. 2022. PMID: 35493787 Free PMC article. Review.
-
Extracellular Vesicles in the Oviduct: Progress, Challenges and Implications for the Reproductive Success.Bioengineering (Basel). 2019 Apr 12;6(2):32. doi: 10.3390/bioengineering6020032. Bioengineering (Basel). 2019. PMID: 31013857 Free PMC article. Review.
Cited by
-
An interactive analysis of the mouse oviductal miRNA profiles.Front Cell Dev Biol. 2022 Oct 19;10:1015360. doi: 10.3389/fcell.2022.1015360. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36340025 Free PMC article.
-
The embryo non-invasive pre-implantation diagnosis era: how far are we?Anim Reprod. 2023 Sep 4;20(2):e20230069. doi: 10.1590/1984-3143-AR2023-0069. eCollection 2023. Anim Reprod. 2023. PMID: 37720726 Free PMC article.
-
The bovine oviductal environment and composition are negatively affected by elevated body energy reserves.PLoS One. 2025 Jun 23;20(6):e0326138. doi: 10.1371/journal.pone.0326138. eCollection 2025. PLoS One. 2025. PMID: 40549708 Free PMC article.
-
Different seminal ejaculated fractions in artificial insemination condition the protein cargo of oviductal and uterine extracellular vesicles in pig.Front Cell Dev Biol. 2023 Oct 6;11:1231755. doi: 10.3389/fcell.2023.1231755. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37868907 Free PMC article.
-
Spermatozoa, acts as an external cue and alters the cargo and production of the extracellular vesicles derived from oviductal epithelial cells in vitro.J Cell Commun Signal. 2023 Sep;17(3):737-755. doi: 10.1007/s12079-022-00715-w. Epub 2022 Dec 5. J Cell Commun Signal. 2023. PMID: 36469292 Free PMC article.
References
-
- Avilés M, Coy P, Rizos D. The oviduct: a key organ for the success of early reproductive events. Anim Front. (2015) 5:25–31. 10.2527/af.2015-0005 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

