"COVID arm": A reaction to the Moderna vaccine
- PMID: 33748377
- PMCID: PMC7959672
- DOI: 10.1016/j.jdcr.2021.02.014
"COVID arm": A reaction to the Moderna vaccine
Keywords: COVID-19; Moderna; general dermatology; medical dermatology; reaction; vaccine.
Conflict of interest statement
Dr Lebwohl is an employee of Mount Sinai Dermatology and receives research funds from 10.13039/100006483Abbvie, 10.13039/100002429Amgen, Eli 10.13039/100004312Lilly, 10.13039/100005205Janssen Research & Development, LLC, 10.13039/100004336Novartis, Ortho Dermatologics, Regeneron-10.13039/100004339Sanofi, and 10.13039/100011110UCB, Inc. He has been the principal investigator in numerous clinical trials but has no personal financial gain. Authors Wei, Fishman, Wattenberg, and Gordon have no conflicts of interest to declare.
Figures
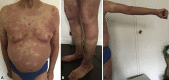
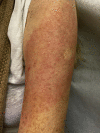


References
-
- Clinical Trial Data Moderna COVID-19 Vaccine (EUA) https://www.modernatx.com/covid19vaccine-eua/providers/clinical-trial-data Available at:
-
- Usatoday.com. 2021. https://www.usatoday.com/story/news/health/2021/01/27/covid-arm-moderna-... Available at:
-
- Cdc.gov. 2021. Reactions and adverse events of the Pfizer-BioNTech COVID-19 vaccine | CDC. 2021. https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenic... Available at:
-
- Moderna COVID-19 vaccine EUA fact sheet for recipients and caregivers (n.d.) https://www.fda.gov/media/144638/download Available at:
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
