Coronary lipid-rich plaque characteristics in Japanese patients with acute coronary syndrome and stable angina: A near infrared spectroscopy and intravascular ultrasound study
- PMID: 33748401
- PMCID: PMC7957086
- DOI: 10.1016/j.ijcha.2021.100747
Coronary lipid-rich plaque characteristics in Japanese patients with acute coronary syndrome and stable angina: A near infrared spectroscopy and intravascular ultrasound study
Abstract
Background: Asians have a much lower incidence of adverse coronary events than Caucasians. We sought to evaluate the characteristics of coronary lipid-rich plaques (LRP) in Asian patients with acute coronary syndrome (ACS) and stable angina (SA). We also aimed to identify surrogate markers for the extent of LRP.
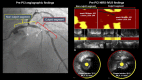
Methods: We evaluated 207 patients (ACS, n = 75; SA, n = 132) who underwent percutaneous coronary intervention under near infrared spectroscopy intravascular ultrasound (NIRS-IVUS). Plaque characteristics and the extent of LRP [defined as a long segment with a 4-mm maximum lipid-core burden index (maxLCBI4mm)] on NIRS in de-novo culprit and non-culprit segments were analyzed.
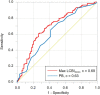
Results: The ACS culprit lesions had a significantly higher maxLCBI4mm (median [interquartile range (IQR)]: 533 [385-745] vs. 361 [174-527], p < 0.001) than the SA culprit lesions. On multivariate logistic analysis, a large LRP (defined as maxLCBI4mm ≥ 400) was the strongest independent predictor of the ACS culprit segment (odds ratio, 3.87; 95% confidence interval, 1.95-8.02). In non-culprit segments, 19.8% of patients had at least one large LRP without a small lumen. No significant correlation was found between the extent of LRP and systematic biomarkers (hs-CRP, IL-6, TNF-α), whereas the extent of LRP was positively correlated with IVUS plaque burden (r = 0.24, p < 0.001).
Conclusions: We confirmed that NIRS-IVUS plaque assessment could be useful to differentiate ACS from SA culprit lesions, and that a threshold maxLCBI4mm ≥ 400 was clinically suitable in Japanese patients. No surrogate maker for a high-risk LRP was found; consequently, direct intravascular evaluation of plaque characteristics remains important.
Keywords: ACS, acute coronary syndrome; Asian; CI, confidence interval; CKD, chronic kidney disease; IL-6, interleukin-6; IQR, interquartile range; IVUS, intravascular ultrasound; Intracoronary imaging; LCBI; LCBI, lipid core burden index; LDL-C, low-density lipoprotein cholesterol; LRP, lipid-rich plaque; Lipid core burden index; MDA-LDL, malondialdehyde-modified LDL; MLA, minimum lumen area; NIRS; NIRS, near infrared spectroscopy; NSTE-ACS, non-ST elevation acute coronary syndrome; OR, odds ratio; PCI, percutaneous coronary intervention; PCSK9, proprotein convertase subtilisin / kexin type 9; SA, stable angina; STEMI, ST-elevation myocardial infarction; TNF-α, tumor necrosis factor-α; Vulnerable plaque; hs-CRP, high-sensitive C reactive protein.
© 2021 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Muller J.E., Tofler G.H., Stone P.H. Circadian variation and triggers of onset of acute cardiovascular disease. Circulation. 1989;79:733–743. - PubMed
-
- Falk E., Nakano M., Bentzon J.F., Finn A.V., Virmani R. Update on acute coronary syndromes: the pathologists' view. Eur. Heart J. 2013;34:719–728. - PubMed
-
- Virmani R., Burke A.P., Farb A., Kolodgie F.D. Pathology of the vulnerable plaque. J. Am. Coll. Cardiol. 2006;47:C13–C18. - PubMed
-
- Gardner C.M., Tan H., Hull E.L., Lisauskas J.B., Sum S.T., Meese T.M. Detection of lipid core coronary plaques in autopsy specimens with a novel catheter-based near-infrared spectroscopy system. JACC Cardiovasc. Imaging. 2008;1:638–648. - PubMed
-
- Moreno P.R., Lodder R.A., Purushothaman K.R., Charash W.E., O'Connor W.N., Muller J.E. Detection of lipid pool, thin fibrous cap, and inflammatory cells in human aortic atherosclerotic plaques by near-infrared spectroscopy. Circulation. 2002;105:923–927. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

