Ex Vivo Perfusion With Methylprednisolone Attenuates Brain Death-induced Lung Injury in Rats
- PMID: 33748411
- PMCID: PMC7969243
- DOI: 10.1097/TXD.0000000000001141
Ex Vivo Perfusion With Methylprednisolone Attenuates Brain Death-induced Lung Injury in Rats
Abstract
The onset of brain death (BD) leads to the deterioration of potential donor lungs. Methylprednisolone is considered to increase lung oxygenation capacity and enhance the procurement yield of donor lungs, when applied in situ, during donor management. However, whether BD-induced lung damage is ameliorated upon treatment with methylprednisolone during acellular ex vivo lung perfusion (EVLP), remains unknown. We aimed to investigate whether the quality of lungs from brain-dead donors improves upon methylprednisolone treatment during EVLP.
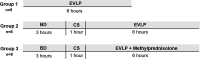
Methods: Rat lungs were randomly assigned to 1 of 3 experimental groups (n = 8/group): (1) healthy, directly procured lungs subjected to EVLP; (2) lungs from brain-dead rats subjected to cold storage and EVLP; and (3) lungs from brain-dead rats subjected to cold storage and EVLP with 40 mg methylprednisolone added to the perfusate. Ventilation and perfusion parameters, histology, edema formation, metabolic profile, and inflammatory status of lungs were investigated.
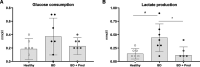
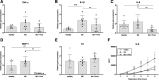
Results: Methylprednisolone treated lungs from brain-dead donors improved positive inspiratory pressures needed to maintain tidal volumes of 7 mL/kg of body weight, which was 25.6 ± 5.8 cm H2O in untreated lungs and 18.0 ± 3.0 cm H2O in methylprednisolone treated lungs, after 6 h EVLP. Furthermore, dynamic lung compliance increased upon methylprednisolone treatment, with values of 0.11 ± 0.05 mL/cm H2O versus 0.18 ± 0.04 mL/cm H2O after 6 h of EVLP. Methylprednisolone treatment ameliorated the amount of lung edema, as corroborated by a reduction of 0.7 in the wet/dry ratio. Although glucose consumption levels were comparable, the BD-induced cumulative lactate production decreased from 0.44 ± 0.26 to 0.11 ± 0.16 mmol/L upon methylprednisolone treatment. Finally, BD-induced inflammatory status was reduced upon methylprednisolone treatment compared to untreated lungs from brain-dead donors, as reflected by lower proinflammatory gene expression levels of IL-1β, IL-6 and MCP-1, and IL-6 perfusate levels.
Conclusions: We showed that methylprednisolone treatment during EVLP attenuates BD-induced lung injury.
Copyright © 2021 The Author(s). Transplantation Direct. Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors declare no funding or conflicts of interest.
Figures


Similar articles
-
A translational rat model for ex vivo lung perfusion of pre-injured lungs after brain death.PLoS One. 2021 Dec 2;16(12):e0260705. doi: 10.1371/journal.pone.0260705. eCollection 2021. PLoS One. 2021. PMID: 34855870 Free PMC article.
-
Male versus female inflammatory response after brain death model followed by ex vivo lung perfusion.Biol Sex Differ. 2024 Jan 29;15(1):11. doi: 10.1186/s13293-024-00581-8. Biol Sex Differ. 2024. PMID: 38287395 Free PMC article.
-
Endothelin receptor antagonist improves donor lung function in an ex vivo perfusion system.J Biomed Sci. 2020 Oct 2;27(1):96. doi: 10.1186/s12929-020-00690-7. J Biomed Sci. 2020. PMID: 33008372 Free PMC article.
-
North American expert consensus on the clinical role of ex vivo lung perfusion (EVLP) with acellular perfusate.J Thorac Dis. 2025 Apr 30;17(4):1832-1843. doi: 10.21037/jtd-2024-2069. Epub 2025 Apr 27. J Thorac Dis. 2025. PMID: 40400975 Free PMC article. Review.
-
The Conversional Efficacy of Ex Vivo Lung Perfusion and Clinical Outcomes in Patients Undergoing Transplantation of Donor Lungs by Ex Vivo Lung Perfusion: A Meta-Analysis.Ann Transplant. 2019 Dec 27;24:647-660. doi: 10.12659/AOT.919242. Ann Transplant. 2019. PMID: 31879416 Free PMC article.
Cited by
-
Low-Volume Ex Situ Lung Perfusion System for Single Lung Application in a Small Animal Model Enables Optimal Compliance With "Reduction" in 3R Principles of Animal Research.Transpl Int. 2024 Sep 9;37:13189. doi: 10.3389/ti.2024.13189. eCollection 2024. Transpl Int. 2024. PMID: 39314923 Free PMC article.
-
Experimental Models of Ischemic Lung Damage for the Study of Therapeutic Reconditioning During Ex Vivo Lung Perfusion.Transplant Direct. 2022 Jun 10;8(7):e1337. doi: 10.1097/TXD.0000000000001337. eCollection 2022 Jul. Transplant Direct. 2022. PMID: 35702630 Free PMC article.
-
Ex vivo lung perfusion and the Organ Care System: a review.Clin Transplant Res. 2024 Mar 31;38(1):23-36. doi: 10.4285/ctr.23.0057. Clin Transplant Res. 2024. PMID: 38725180 Free PMC article. Review.
-
Inflammation and Oxidative Stress in the Context of Extracorporeal Cardiac and Pulmonary Support.Front Immunol. 2022 Mar 4;13:831930. doi: 10.3389/fimmu.2022.831930. eCollection 2022. Front Immunol. 2022. PMID: 35309362 Free PMC article. Review.
-
Ex Vivo Lung Perfusion: A Review of Current and Future Application in Lung Transplantation.Pulm Ther. 2022 Jun;8(2):149-165. doi: 10.1007/s41030-022-00185-w. Epub 2022 Mar 22. Pulm Ther. 2022. PMID: 35316525 Free PMC article. Review.
References
-
- Eurotransplant. Annual Report. 2019. Avaliable at https://www.eurotransplant.org/wp-content/uploads/2020/06/Annual-Report-.... Accessed July 23, 2020.
-
- Avlonitis VS, Wigfield CH, Kirby JA, et al. . The hemodynamic mechanisms of lung injury and systemic inflammatory response following brain death in the transplant donor. Am J Transplant. 2005;5(4 Pt 1):684–693. - PubMed
-
- Cypel M, Yeung JC, Liu M, et al. . Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med. 2011;364:1431–1440. - PubMed
-
- Follette DM, Rudich SM, Babcock WD. Improved oxygenation and increased lung donor recovery with high-dose steroid administration after brain death. J Heart Lung Transplant. 1998;17:423–429. - PubMed
-
- Dupuis S, Amiel JA, Desgroseilliers M, et al. . Corticosteroids in the management of brain-dead potential organ donors: a systematic review. Br J Anaesth. 2014;113:346–359. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

