A novel immuno-oncology algorithm measuring tumor microenvironment to predict response to immunotherapies
- PMID: 33748492
- PMCID: PMC7970145
- DOI: 10.1016/j.heliyon.2021.e06438
A novel immuno-oncology algorithm measuring tumor microenvironment to predict response to immunotherapies
Abstract
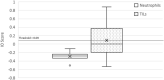
Immune checkpoint inhibitor (ICI) therapies can improve clinical outcomes for patients with solid tumors, but relatively few patients respond. Because ICI therapies support an adaptive immune response, patients with an active tumor microenvironment (TME) may be more likely to respond, and thus biomarkers capable of discerning an active from a quiescent TME may be useful in patient selection. We developed an algorithm optimized for genes expressed in the mesenchymal and immunomodulatory subtypes of a 101-gene triple negative breast cancer model (Ring, BMC Cancer, 2016, 16:143) as a means to capture the immunological state of the TME. We compared the outcome of the algorithm (IO score) with the 101-gene model and found 88% concordance, indicating the models are correlated but not identical, and may be measuring different TME features. We found 92.5% correlation between IO scores of matched tumor epithelial and adjacent stromal tissues, indicating the IO score is not specific to these tissues, but reflects the TME as a whole. We observed a significant difference in IO score (p = 0.0092) between samples with high tumor-infiltrating lymphocytes and samples with increased neutrophil load, demonstrating agreement between IO score and these two prognostic markers. Finally, among non-small cell lung cancer patients receiving immunotherapy, we observed a significant difference in IO score (p = 0.0035) between responders and non-responders, and a significant odds ratio (OR = 5.76, 95% CI 1.30-25.51, p = 0.021), indicating the IO score can predict patient response. The immuno-oncology algorithm may offer independent and incremental predictive value over current biomarkers in the clinic.
Keywords: Biomarker; Immune checkpoint inhibitors; Immunotherapy; NSCLC; TNBC; Tumor microenvironment.
© 2021 The Author(s).
Conflict of interest statement
T.J.N., B.L.S., and D.R.H. are employed by Oncocyte Corporation, the commercial entity that markets the immuno-oncology algorithm as DetermaIO®. B.Z.R. and R.S.S. are consultants contracted by Oncocyte Corporation. We have filed a provisional patent around some of the work cited in this manuscript.
Figures



Similar articles
-
Translation of the 27-gene immuno-oncology test (IO score) to predict outcomes in immune checkpoint inhibitor treated metastatic urothelial cancer patients.J Transl Med. 2022 Aug 16;20(1):370. doi: 10.1186/s12967-022-03563-9. J Transl Med. 2022. PMID: 35974414 Free PMC article.
-
Next generation immuno-oncology tumor profiling using a rapid, non-invasive, computational biophysics biomarker in early-stage breast cancer.Front Artif Intell. 2023 Apr 17;6:1153083. doi: 10.3389/frai.2023.1153083. eCollection 2023. Front Artif Intell. 2023. PMID: 37138891 Free PMC article.
-
New Immuno-oncology Targets and Resistance Mechanisms.Curr Treat Options Oncol. 2022 Sep;23(9):1201-1218. doi: 10.1007/s11864-022-01005-8. Epub 2022 Aug 18. Curr Treat Options Oncol. 2022. PMID: 35980521 Review.
-
Recent Trends and Opportunities for the Targeted Immuno-Nanomaterials for Cancer Theranostics Applications.Micromachines (Basel). 2022 Dec 14;13(12):2217. doi: 10.3390/mi13122217. Micromachines (Basel). 2022. PMID: 36557516 Free PMC article. Review.
-
Multifunctional Nanocarriers-Mediated Synergistic Combination of Immune Checkpoint Inhibitor Cancer Immunotherapy and Interventional Oncology Therapy.Adv Nanobiomed Res. 2021 Oct;1(10):2100010. doi: 10.1002/anbr.202100010. Epub 2021 Aug 2. Adv Nanobiomed Res. 2021. PMID: 35663354 Free PMC article.
Cited by
-
Cancer Immunotherapy with Immune Checkpoint Inhibitors-Biomarkers of Response and Toxicity; Current Limitations and Future Promise.Diagnostics (Basel). 2022 Jan 6;12(1):124. doi: 10.3390/diagnostics12010124. Diagnostics (Basel). 2022. PMID: 35054292 Free PMC article. Review.
-
Biomarkers of response and resistance to immune checkpoint inhibitors in breast cancer.Breast. 2025 Jul 21;83:104545. doi: 10.1016/j.breast.2025.104545. Online ahead of print. Breast. 2025. PMID: 40716359 Free PMC article. Review.
-
Pharmacological Mechanism of Mume Fructus in the Treatment of Triple-Negative Breast Cancer Based on Network Pharmacology.Appl Biochem Biotechnol. 2024 Nov;196(11):7974-7993. doi: 10.1007/s12010-024-04948-w. Epub 2024 Apr 26. Appl Biochem Biotechnol. 2024. PMID: 38668843
-
The 27-gene IO score is associated with efficacy of PD-1/L1 inhibitors independent of FGFR expression in a real-world metastatic urothelial carcinoma cohort.Cancer Immunol Immunother. 2023 Jul;72(7):2075-2086. doi: 10.1007/s00262-023-03401-x. Epub 2023 Feb 19. Cancer Immunol Immunother. 2023. PMID: 36806983 Free PMC article.
-
Characterization of the tumor immune-microenvironment of adenocarcinoma of lung with a metastatic lesion in the pancreas treated successfully with first-line, single-agent pembrolizumab.Ther Adv Med Oncol. 2021 Apr 19;13:17588359211010156. doi: 10.1177/17588359211010156. eCollection 2021. Ther Adv Med Oncol. 2021. PMID: 33953802 Free PMC article.
References
-
- Tang J., Shalabi A., Hubbard-Lucey V.M. Comprehensive analysis of the clinical immuno-oncology landscape. Ann. Oncol. 2018;29(1):84–91. - PubMed
-
- Postow M.A., Sidlow R., Hellmann M.D. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 2018;378(2):158–168. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

