Preanalytical Issues and Cycle Threshold Values in SARS-CoV-2 Real-Time RT-PCR Testing: Should Test Results Include These?
- PMID: 33748564
- PMCID: PMC7970463
- DOI: 10.1021/acsomega.1c00166
Preanalytical Issues and Cycle Threshold Values in SARS-CoV-2 Real-Time RT-PCR Testing: Should Test Results Include These?
Abstract
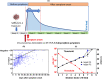
Since the emergence of SARS-CoV-2 pandemic, clinical laboratories worldwide are overwhelmed with SARS-CoV-2 testing using the current gold standard: real-time reverse-transcription polymerase chain reaction (RT-PCR) assays. The large numbers of suspected cases led to shortages in numerous reagents such as specimen transport and RNA extraction buffers. We try to provide some answers on how strongly preanalytical issues affect RT-PCR results by reviewing the utility of different transport buffer media and virus inactivation procedures and comparing the literature data with our own recent findings. We show that various viral inactivation procedures and transport buffers are available and are less of a bottleneck for PCR-based methods. However, efficient alternative lysis buffers remain more difficult to find, and several fast RT-PCR assays are not compatible with guanidine-containing media, making this aspect more of a challenge in the current crisis. Furthermore, the availability of different SARS-CoV-2-specific RT-PCR kits with different sensitivities makes the definition of a general cutoff level for the cycle threshold (Ct) value challenging. Only a few studies have considered how Ct values relate to viral infectivity and how preanalytical issues might affect viral infectivity and RNA detection. We review the current data on the correlation between Ct values and viral infectivity. The presence of the SARS-CoV-2 viral genome in its own is not sufficient proof of infectivity and caution is needed in evaluation of the infectivity of samples. The correlation between Ct values and viral infectivity revealed an RT-PCR cutoff value of 34 cycles for SARS-CoV-2 infectivity using a laboratory-developed RT-PCR assay targeting the RdRp gene. While ideally each clinical laboratory should perform its own correlation, we believe this perspective article could be a reference point for others, in particular medical doctors and researchers interested in COVID-19 diagnostics, and a first step toward harmonization.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Voysey M.; Costa Clemens S. A.; Madhi S.; Weckx L. Y.; Folegatti P. M.; Aley P. K.; Angus B.; VBaillie V. L.; Barnabas S. L.; Bhorat Q. E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2020, 397, 99–111. 10.1016/S0140-6736(20)32661-1. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

