Novel Multifunctional Samarium-Organic Framework for Fluorescence Sensing of Ag+, MnO4 -, and Cimetidine and Electrochemical Sensing of o-Nitrophenol in Aqueous Solutions
- PMID: 33748594
- PMCID: PMC7970500
- DOI: 10.1021/acsomega.0c05867
Novel Multifunctional Samarium-Organic Framework for Fluorescence Sensing of Ag+, MnO4 -, and Cimetidine and Electrochemical Sensing of o-Nitrophenol in Aqueous Solutions
Abstract
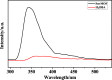
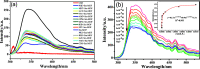
A novel Sm-metal-organic framework (MOF) sensor with the molecular formula Sm8(HDBA)6·H2O has been prepared based on a penta-carboxyl organic ligand (H5DBA = 3,5-di(2',4'-dicarboxylphenyl)benzoic acid) and samarium nitrate under solvothermal conditions. Sm-MOF is characterized by single-crystal X-ray diffraction analysis, elemental analysis, thermogravimetric analysis, and powder X-ray diffraction analysis. Structural analysis shows that the dimer metal units are alternately connected to form a one-dimensional chain, and this chain is connected by the bridging carboxyl oxygen of the ligand H5DBA to form a two-dimensional double-layer plane, which then expands into a three-dimensional microporous framework. Fluorescence detection studies show that Sm-MOF can detect Ag+ ions, MnO4 - anions, and cimetidine tablets with high sensitivity and selectivity and can also be used to electrochemically detect o-nitrophenol in water. High-sensitivity detection capability of the Sm-MOF can enrich the application of samarium complexes in multifunctional sensors.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
A Multifunctional Tb-MOF Detector for H2O2, Fe3+, Cr2O7 2-, and TPA Explosive Featuring Coexistence of Binuclear and Tetranuclear Clusters.ACS Omega. 2020 Dec 14;5(51):33039-33046. doi: 10.1021/acsomega.0c04526. eCollection 2020 Dec 29. ACS Omega. 2020. PMID: 33403265 Free PMC article.
-
Stable Interpenetrated Zirconium-Based Metal-Organic Framework for the Fluorescence Detection of MnO4.Inorg Chem. 2025 Apr 7;64(13):6648-6655. doi: 10.1021/acs.inorgchem.5c00200. Epub 2025 Mar 24. Inorg Chem. 2025. PMID: 40128184
-
A novel microporous Tb-MOF fluorescent sensor for highly selective and sensitive detection of picric acid.RSC Adv. 2018 Jun 13;8(39):21671-21678. doi: 10.1039/c8ra02602e. eCollection 2018 Jun 13. RSC Adv. 2018. PMID: 35541725 Free PMC article.
-
Heterometallic Alkaline Earth-Lanthanide Ba(II)-La(III) Microporous Metal-Organic Framework as Bifunctional Luminescent Probes of Al(3+) and MnO4(.).Inorg Chem. 2016 May 2;55(9):4391-402. doi: 10.1021/acs.inorgchem.6b00111. Epub 2016 Apr 18. Inorg Chem. 2016. PMID: 27088966
-
Metal-Organic Framework-Based Sensors for Environmental Contaminant Sensing.Nanomicro Lett. 2018;10(4):64. doi: 10.1007/s40820-018-0218-0. Epub 2018 Jul 13. Nanomicro Lett. 2018. PMID: 30393712 Free PMC article. Review.
Cited by
-
Fluorescence-Responsive Detection of Ag(I), Al(III), and Cr(III) Ions Using Cd(II) Based Pillared-Layer Frameworks.Int J Mol Sci. 2022 Dec 26;24(1):369. doi: 10.3390/ijms24010369. Int J Mol Sci. 2022. PMID: 36613812 Free PMC article.
-
Explicating the recognition phenomenon of hazardous nitro-aromatic compound from contaminated environmental and cellular matrices by rationally designed pyridine-functionalized molecular probes.Heliyon. 2023 Feb 9;9(2):e13620. doi: 10.1016/j.heliyon.2023.e13620. eCollection 2023 Feb. Heliyon. 2023. PMID: 36873140 Free PMC article.
-
Recent Advancements in Sensing of Silver ions by Different Host Molecules: An Overview (2018-2023).J Fluoresc. 2025 Jan;35(1):267-289. doi: 10.1007/s10895-023-03494-8. Epub 2023 Dec 1. J Fluoresc. 2025. PMID: 38038876 Review.
-
Lanthanide-MOFs as multi-responsive photoluminescence sensor for sensitively detecting Fe3+, Cr2O72- and nitrofuran antibiotics.RSC Adv. 2023 Sep 4;13(37):26196-26202. doi: 10.1039/d3ra03817c. eCollection 2023 Aug 29. RSC Adv. 2023. PMID: 37671001 Free PMC article.
References
-
- Mukherjee S.; Ganguly S.; Samanta D.; Das D. Sustainable Green Route to Synthesize Functional Nano-MOFs as Selective Sensing Probes for CrVI Oxoanions and as Specific Sequestering Agents for Cr2O72–. ACS Sustainable Chem. Eng. 2020, 8, 1195–1206. 10.1021/acssuschemeng.9b06393. - DOI
-
- He J.; Xu J.-L.; Yin J.-C.; Li N.; Bu X.-H. Recent advances in luminescent metal-organic frameworks for chemical sensors. Sci. China Mater. 2019, 62, 1655–1678. 10.1007/s40843-019-1169-9. - DOI
-
- Lyle S.-J.; Waller P.-J.; Yaghi O.-M. Covalent organic frameworks: Organic chemistry extended into two and three dimensions. Trends Chem. 2019, 1, 172–184. 10.1016/j.trechm.2019.03.001. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

