Comparative Study of Human Pluripotent Stem Cell-Derived Endothelial Cells in Hydrogel-Based Culture Systems
- PMID: 33748608
- PMCID: PMC7970572
- DOI: 10.1021/acsomega.0c06187
Comparative Study of Human Pluripotent Stem Cell-Derived Endothelial Cells in Hydrogel-Based Culture Systems
Abstract
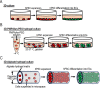
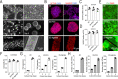
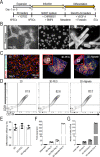
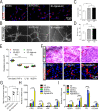
Human pluripotent stem cell (hPSC)-derived endothelial cells (ECs) are promising cell sources for drug discovery, tissue engineering, and studying or treating vascular diseases. However, hPSC-ECs derived from different culture methods display different phenotypes. Herein, we made a detailed comparative study of hPSC-ECs from three different culture systems (e.g., 2D, 3D PNIPAAm-PEG hydrogel, and 3D alginate hydrogel cultures) based on our previous reports. We expanded hPSCs and differentiated them into ECs in three culture systems. Both 3D hydrogel systems could mimic an in vivo physiologically relevant microenvironment to protect cells from shear force and prevent cell agglomeration, leading to a high culture efficiency and a high volumetric yield. We demonstrated that hPSC-ECs produced from both hydrogel systems had similar results as 2D-ECs. The transcriptome analysis showed that PEG-ECs and alginate-ECs displayed a functional phenotype due to their higher gene expressions in vasculature development, extracellular matrix, angiogenesis, and glycolysis, while 2D-ECs showed a proliferative phenotype due to their higher gene expressions in cell proliferation. Taken together, both PEG- and alginate-hydrogel systems will significantly advance the applications of hPSC-ECs in various biomedical fields.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Comparative study of differentiating human pluripotent stem cells into vascular smooth muscle cells in hydrogel-based culture methods.Regen Ther. 2022 Dec 21;22:39-49. doi: 10.1016/j.reth.2022.12.001. eCollection 2023 Mar. Regen Ther. 2022. PMID: 36618488 Free PMC article.
-
A Scalable and Efficient Bioprocess for Manufacturing Human Pluripotent Stem Cell-Derived Endothelial Cells.Stem Cell Reports. 2018 Aug 14;11(2):454-469. doi: 10.1016/j.stemcr.2018.07.001. Epub 2018 Aug 2. Stem Cell Reports. 2018. PMID: 30078557 Free PMC article.
-
Manufacturing human pluripotent stem cell derived endothelial cells in scalable and cell-friendly microenvironments.Biomater Sci. 2018 Dec 18;7(1):373-388. doi: 10.1039/c8bm01095a. Biomater Sci. 2018. PMID: 30484784
-
Generation of Human Pluripotent Stem Cell-derived Endothelial Cells and Their Therapeutic Utility.Curr Cardiol Rep. 2018 May 5;20(6):45. doi: 10.1007/s11886-018-0985-8. Curr Cardiol Rep. 2018. PMID: 29730842 Free PMC article. Review.
-
Review: Human stem cell-based 3D in vitro angiogenesis models for preclinical drug screening applications.Mol Biol Rep. 2024 Feb 1;51(1):260. doi: 10.1007/s11033-023-09048-2. Mol Biol Rep. 2024. PMID: 38302762 Free PMC article. Review.
Cited by
-
Comparative study of differentiating human pluripotent stem cells into vascular smooth muscle cells in hydrogel-based culture methods.Regen Ther. 2022 Dec 21;22:39-49. doi: 10.1016/j.reth.2022.12.001. eCollection 2023 Mar. Regen Ther. 2022. PMID: 36618488 Free PMC article.
-
Fabricating 3-dimensional human brown adipose microtissues for transplantation studies.Bioact Mater. 2022 Oct 27;22:518-534. doi: 10.1016/j.bioactmat.2022.10.022. eCollection 2023 Apr. Bioact Mater. 2022. PMID: 36330162 Free PMC article.
-
Carrageenan maintains the contractile phenotype of vascular smooth muscle cells by increasing macromolecular crowding in vitro.Eur J Med Res. 2024 Apr 22;29(1):249. doi: 10.1186/s40001-024-01843-2. Eur J Med Res. 2024. PMID: 38650027 Free PMC article.
-
Application of vascular endothelial cells in stem cell medicine.World J Clin Cases. 2021 Dec 16;9(35):10765-10780. doi: 10.12998/wjcc.v9.i35.10765. World J Clin Cases. 2021. PMID: 35047589 Free PMC article. Review.
-
Improving three-dimensional human pluripotent cell culture efficiency via surface molecule coating.Front Chem Eng. 2022;4:1031395. doi: 10.3389/fceng.2022.1031395. Epub 2022 Oct 20. Front Chem Eng. 2022. PMID: 40677628 Free PMC article.
References
-
- Patsch C.; Challet-Meylan L.; Thoma E. C.; Urich E.; Heckel T.; O’Sullivan J. F.; Grainger S. J.; Kapp F. G.; Sun L.; Christensen K.; Xia Y.; Florido M. H. C.; He W.; Pan W.; Prummer M.; Warren C. R.; Jakob-Roetne R.; Certa U.; Jagasia R.; Freskgård P.-O.; Adatto I.; Kling D.; Huang P.; Zon L. I.; Chaikof E. L.; Gerszten R. E.; Graf M.; Iacone R.; Cowan C. a. Generation of Vascular Endothelial and Smooth Muscle Cells from Human Pluripotent Stem Cells. Nat. Cell Biol. 2015, 17, 994–1003. 10.1038/ncb3205. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

