Synthesis of Diaminopyrimidine Sulfonate Derivatives and Exploration of Their Structural and Quantum Chemical Insights via SC-XRD and the DFT Approach
- PMID: 33748618
- PMCID: PMC7970555
- DOI: 10.1021/acsomega.0c06323
Synthesis of Diaminopyrimidine Sulfonate Derivatives and Exploration of Their Structural and Quantum Chemical Insights via SC-XRD and the DFT Approach
Abstract
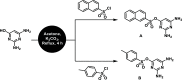
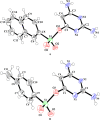
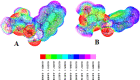
Two heterocyclic compounds named 2,6-diaminopyrimidin-4-ylnaphthalene-2-sulfonate (A) and 2,6-diaminopyrimidin-4-yl4-methylbenzene sulfonate (B) were synthesized. The structures of heterocyclic molecules were established by the X-ray crystallographic technique, which showed several noncovalent interactions as N···H···N, N···H···O, and C-H···O bonding and parallel offset stacking interaction. Hydrogen-bonding interactions were further explored by the Hirshfeld surface (HS) analysis. Nonlinear optical (NLO) and natural bond orbital (NBO) properties were calculated utilizing the B3LYP/6-311G(d,p) level. Frontier molecular orbitals (FMOs) and molecular electrostatic potential (MEP) were calculated utilizing the time-dependent density functional theory (TD-DFT) at the same level. The NBO analysis showed that the molecular stabilities of compounds A and B were attributed to their large stabilization energy values. The second hyperpolarizability (γtot) values for A and B were obtained as 3.7 × 104 and 2.7 × 104 au, respectively. The experimental X-ray crystallographic and theoretical structural parameters of A and B were found to be in close correspondence. Both the molecules reveal substantial NLO responses that can be significant for their utilization in advanced applications.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Nagaraj A.; Reddy C. S. Synthesis and biological study of novel bis-chalcones, bis-thiazines and bis-pyrimidines. J. Iran. Chem. Soc. 2008, 5, 262–267. 10.1007/BF03246116. - DOI
-
- Ajani O. O.; Isaac J. T.; Owoeye T. F.; Akinsiku A. A. Exploration of the chemistry and biological properties of pyrimidine as a privilege pharmacophore in therapeutics. Int. J. Biol. Chem. 2015, 9, 148–177. 10.3923/ijbc.2015.148.177. - DOI
-
- Rao N. V.; Vaisalini N B.; Mounika B.; Harika V. L.; Desu P. K.; Nama S. An overview on synthesis and biological activity of pyrimidines. Int. J. Pharm. Chem. Res. 2013, 2, 14–22.
-
- Kim J. M.; Kim S. H.; Kim J.-N. Synthesis of 2, 4, 5-trisubstituted pyrimidines from Baylis-Hillman adducts and amidines. Bull. Korean Chem. Soc. 2007, 28, 2505–2507. 10.5012/bkcs.2007.28.12.2505. - DOI
-
- Almansa C.; de Arriba A. F.; Cavalcanti F. L.; Gómez L. A.; Miralles A.; Merlos M.; García-Rafanell J.; Forn J. Synthesis and SAR of a new series of COX-2-selective inhibitors: pyrazolo [1, 5-a] pyrimidines. J. Med. Chem. 2001, 44, 350–361. 10.1021/jm0009383. - DOI - PubMed
- Gupta J. K.; Chaudhary A.; Dudhe R.; Varuna K.; Sharma P.; Verma P. A review on the synthesis and therapeutic potential of pyrimidine derivatives. Int. J. Pharma. Sci. Res 2010, 1, 34–49.
- Asobo P. F.; Wahe H.; Mbafor J. T.; Nkengfack A. E.; Fomum Z. T.; Sopbue E. F.; Dopp D. Heterocycles of biological importance. Part 5. The formation of novel biologically active pyrimido [1, 2-a] benzimidazoles from allenic nitriles and aminobenzimidazoles. J. Chem. Soc., Perkin Trans. 1 2001, 1, 457–461. 10.1039/b005511p. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

