Copper Nanoparticle Loading and F Doping of Graphene Aerogel Enhance Its Adsorption of Aqueous Perfluorooctanoic Acid
- PMID: 33748621
- PMCID: PMC7970550
- DOI: 10.1021/acsomega.1c00044
Copper Nanoparticle Loading and F Doping of Graphene Aerogel Enhance Its Adsorption of Aqueous Perfluorooctanoic Acid
Abstract
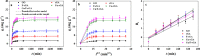
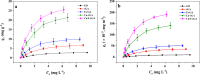
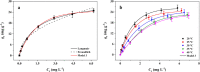
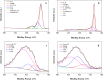
Perfluorooctanoic acid (PFOA) persists in the environment for a long time due to its stable physical and chemical properties, and it is harmful to the environment and biological system. In order to effectively remove PFOA from aqueous solution, Cu nanoparticles and fluorine-modified graphene aerogel (Cu/F-rGA) were fabricated by the microbubble template method. Compared with unmodified aerogels (rGA), the adsorption rate of PFOA on Cu/F-rGA was enhanced 2.68-fold. These significant improvements were assumed to benefit from the ligand exchange reaction and hydrophobic and F-F interactions. The regeneration of Cu/F-rGA maintained 73.26% with ethanol as the desorption solvent after 10 times adsorption-desorption. The fitting results of the statistical physics model showed that PFOA tended to be parallel to the adsorption site at low temperature and perpendicular at high temperature. The number of PFOA molecules connected to each adsorption site was 0.53 to 1.41, and the number of adsorption layers of PFOA on the Cu/F-rGA was between 1.63 and 2.51. Compared with the response surface methodology and artificial neural network, an adaptive neuro-fuzzy inference system had more accurate analysis and prediction results. These results provide an effective and alternative strategy to remove PFOA from aqueous solution with environment-friendly consumption.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Electrochemical adsorption of perfluorooctanoic acid on a novel reduced graphene oxide aerogel loaded with Cu nanoparticles and fluorine.J Hazard Mater. 2021 Aug 15;416:125866. doi: 10.1016/j.jhazmat.2021.125866. Epub 2021 Apr 12. J Hazard Mater. 2021. PMID: 33894436
-
Removal of perfluorooctanoic acid (PFOA) from aqueous solution by amino-functionalized graphene oxide (AGO) aerogels: Influencing factors, kinetics, isotherms, and thermodynamic studies.Sci Total Environ. 2021 Aug 20;783:147041. doi: 10.1016/j.scitotenv.2021.147041. Epub 2021 Apr 20. Sci Total Environ. 2021. PMID: 34088148
-
TiO2 quantum dots loaded sulfonated graphene aerogel for effective adsorption-photocatalysis of PFOA.Sci Total Environ. 2020 Jan 1;698:134275. doi: 10.1016/j.scitotenv.2019.134275. Epub 2019 Sep 3. Sci Total Environ. 2020. PMID: 31505352
-
Metal nanoparticles by doping carbon nanotubes improved the sorption of perfluorooctanoic acid.J Hazard Mater. 2018 Jun 5;351:206-214. doi: 10.1016/j.jhazmat.2018.03.001. Epub 2018 Mar 8. J Hazard Mater. 2018. PMID: 29550554
-
Photodegradation of perfluorooctanoic acid by graphene oxide-deposited TiO2 nanotube arrays in aqueous phase.J Environ Manage. 2018 Jul 15;218:333-339. doi: 10.1016/j.jenvman.2018.04.016. Epub 2018 Apr 22. J Environ Manage. 2018. PMID: 29689536 Review.
Cited by
-
2D Fluorinated Graphene Oxide (FGO)-Polyethyleneimine (PEI) Based 3D Porous Nanoplatform for Effective Removal of Forever Toxic Chemicals, Pharmaceutical Toxins, and Waterborne Pathogens from Environmental Water Samples.ACS Omega. 2023 Nov 13;8(47):44942-44954. doi: 10.1021/acsomega.3c06360. eCollection 2023 Nov 28. ACS Omega. 2023. PMID: 38046318 Free PMC article.
References
-
- Buck R. C.; Franklin J.; Berger U.; Conder J. M.; Cousins I. T.; De Voogt P.; Jensen A. A.; Kannan K.; Mabury S. A.; van Leeuwen S. P. J. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manage. 2011, 7, 513–541. 10.1002/ieam.258. - DOI - PMC - PubMed
-
- Liu L.; Liu Y.; Gao B.; Ji R.; Li C.; Wang S. Removal of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) from water by carbonaceous nanomaterials: A review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2379–2414. 10.1080/10643389.2019.1700751. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

