Mechanical Biocompatibility, Osteogenic Activity, and Antibacterial Efficacy of Calcium Silicate-Zirconia Biocomposites
- PMID: 33748624
- PMCID: PMC7970563
- DOI: 10.1021/acsomega.1c00097
Mechanical Biocompatibility, Osteogenic Activity, and Antibacterial Efficacy of Calcium Silicate-Zirconia Biocomposites
Abstract
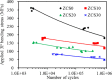
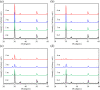
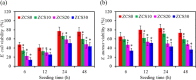
Zirconia ceramics with high mechanical properties have been used as a load-bearing implant in the dental and orthopedic surgery. However, poor bone bonding properties and high elastic modulus remain a challenge. Calcium silicate (CaSi)-based ceramic can foster osteoblast adhesion, growth, and differentiation and facilitate bone ingrowth. This study was to prepare CaSi-ZrO2 composites and evaluate their mechanical properties, long-term stability, in vitro osteogenic activity, and antibacterial ability. The Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) bacteria and human mesenchymal stem cells (hMSCs) were used to evaluate the antibacterial and osteogenic activities of implants in vitro, respectively. Results indicated that the three-point bending strength of ZrO2 was 486 MPa and Young's modulus was 128 GPa, which were much higher than those of the cortical bone. In contrast, the bending strength and modulus of 20% (201 MPa and 48 GPa, respectively) and 30% CaSi (126 MPa and 20 GPa, respectively) composites were close to the reported strength and modulus of the cortical bone. As expected, higher CaSi content implants significantly enhanced cell growth, differentiation, and mineralization of hMSCs. It is interesting to note the induction ability of CaSi in osteogenic differentiation of hMSCs even when cultured in the absence of an osteogenic differentiation medium. The composite with the higher CaSi contents exhibited the greater bacteriostatic effect against E. coli and S. aureus. In conclusion, the addition of 20 wt % CaSi can effectively improve the mechanical biocompatibility, osteogenesis, and antibacterial activity of ZrO2 ceramics, which may be a potential choice for load-bearing applications.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures











Similar articles
-
Long-Term Stability and Osteogenic Activity of Recycled Polysulfone-Calcium Silicate Bone Implants In Vitro.J Funct Biomater. 2025 Jan 17;16(1):31. doi: 10.3390/jfb16010031. J Funct Biomater. 2025. PMID: 39852587 Free PMC article.
-
Electrosprayed calcium silicate nanoparticle-coated titanium implant with improved antibacterial activity and osteogenesis.Colloids Surf B Biointerfaces. 2021 Jun;202:111699. doi: 10.1016/j.colsurfb.2021.111699. Epub 2021 Mar 15. Colloids Surf B Biointerfaces. 2021. PMID: 33743444
-
Calcium Silicate Promoting the Upcycling Potential of Polysulfone Medical Waste in Load-Bearing Applications.J Funct Biomater. 2024 Oct 30;15(11):323. doi: 10.3390/jfb15110323. J Funct Biomater. 2024. PMID: 39590527 Free PMC article.
-
In vitro comparisons of microscale and nanoscale calcium silicate particles.J Mater Chem B. 2020 Jul 22;8(28):6034-6047. doi: 10.1039/d0tb01202e. J Mater Chem B. 2020. PMID: 32597438
-
An overview of zirconia ceramics: basic properties and clinical applications.J Dent. 2007 Nov;35(11):819-26. doi: 10.1016/j.jdent.2007.07.008. Epub 2007 Sep 6. J Dent. 2007. PMID: 17825465 Review.
Cited by
-
Three-Dimensional Printing Methods for Bioceramic-Based Scaffold Fabrication for Craniomaxillofacial Bone Tissue Engineering.J Funct Biomater. 2024 Mar 1;15(3):60. doi: 10.3390/jfb15030060. J Funct Biomater. 2024. PMID: 38535253 Free PMC article. Review.
-
Advances in the Application of Nanomaterials as Treatments for Bacterial Infectious Diseases.Pharmaceutics. 2021 Nov 12;13(11):1913. doi: 10.3390/pharmaceutics13111913. Pharmaceutics. 2021. PMID: 34834328 Free PMC article. Review.
-
Bioactive Glass Modified with Zirconium Incorporation for Dental Implant Applications: Fabrication, Structural, Electrical, and Biological Analysis.Int J Mol Sci. 2023 Jun 24;24(13):10571. doi: 10.3390/ijms241310571. Int J Mol Sci. 2023. PMID: 37445749 Free PMC article.
-
Investigation on the physical properties and biocompatibility of zirconia-alumina-silicate@diopside composite materials and its in vivo toxicity study in embryonic zebrafish.RSC Adv. 2023 Oct 18;13(44):30575-30585. doi: 10.1039/d3ra04555b. eCollection 2023 Oct 18. RSC Adv. 2023. PMID: 37859778 Free PMC article.
-
Biomimetic Scaffolds of Calcium-Based Materials for Bone Regeneration.Biomimetics (Basel). 2024 Aug 24;9(9):511. doi: 10.3390/biomimetics9090511. Biomimetics (Basel). 2024. PMID: 39329533 Free PMC article. Review.
References
-
- Hench L. L. Bioceramics: from concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. 10.1111/j.1151-2916.1991.tb07132.x. - DOI
-
- Yan M.; Csík A.; Yang C.-C.; Luo Y.; Fodor T.; Ding S.-J. Synergistic reinforcement of surface modification on improving the bonding of veneering ceramics to zirconia. Ceram. Int. 2018, 44, 19665–19671. 10.1016/j.ceramint.2018.07.218. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

