Scaling-up of microbial electrosynthesis with multiple electrodes for in situ production of hydrogen peroxide
- PMID: 33748698
- PMCID: PMC7969820
- DOI: 10.1016/j.isci.2021.102094
Scaling-up of microbial electrosynthesis with multiple electrodes for in situ production of hydrogen peroxide
Abstract
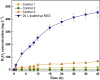
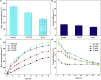
Microbial electrosynthesis system (MES) has recently been shown to be a promising alternative way for realizing in situ and energy-saving synthesis of hydrogen peroxide (H2O2). Although promising, the scaling-up feasibility of such a process is rarely reported. In this study, a 20-L up-scaled two-chamber MES reactor was developed and investigated for in situ and efficient H2O2 electrosynthesis. Maximum H2O2 production rate of 10.82 mg L-1 h-1 and cumulative H2O2 concentration of 454.44 mg L-1 within 42 h were obtained with an input voltage of 0.6 V, cathodic aeration velocity of 0.045 mL min-1 mL-1, 50 mM Na2SO4, and initial pH 3. The electrical energy consumption regarding direct input voltage was only 0.239 kWh kg-1 H2O2, which was further much lower compared with laboratory-scale systems. The obtained results suggested that the future industrialization of MES technology for in situ synthesis of H2O2 and further application in environmental remediation have broad prospects.
Keywords: biotechnology; electrochemistry; engineering; materials science.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- An J., Li N., Zhao Q., Qiao Y., Wang S., Liao C., Zhou L., Li T., Wang X., Feng Y. Highly efficient electro-generation of H2O2 by adjusting liquid-gas-solid three phase interfaces of porous carbonaceous cathode during oxygen reduction reaction. Water Res. 2019;164:114933. - PubMed
-
- Arends J.B., Van Denhouwe S., Verstraete W., Boon N., Rabaey K. Enhanced disinfection of wastewater by combining wetland treatment with bioelectrochemical H2O2 production. Bioresour. Technol. 2014;155:352–358. - PubMed
-
- Attiogbe F.K., Francis R.C. Hydrogen peroxide decomposition in bicarbonate solution catalyzed by ferric citrate. Can. J. Chem. 2011;89:1289–1296.
-
- Brillas E., Maestro A., Moratalla M., Casado J. Electrochemical extraction of oxygen from air via hydroperoxide ion. J. Appl. Electrochem. 1997;27:83–92.
-
- Campos-Martin J.M., Blanco-Brieva G., Fierro J.L. Hydrogen peroxide synthesis: an outlook beyond the anthraquinone process. Angew. Chem. Int. Ed. 2006;45:6962–6984. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

