LIFR-α-dependent adipocyte signaling in obesity limits adipose expansion contributing to fatty liver disease
- PMID: 33748712
- PMCID: PMC7970148
- DOI: 10.1016/j.isci.2021.102227
LIFR-α-dependent adipocyte signaling in obesity limits adipose expansion contributing to fatty liver disease
Abstract
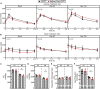
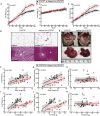
The role of chronic adipose inflammation in diet-induced obesity (DIO) and its sequelae including fatty liver disease remains unclear. Leukemia inhibitory factor (LIF) induces JAK-dependent adipocyte lipolysis and altered adipo/cytokine expression, suppressing in vivo adipose expansion in normal and obese mouse models. To characterize LIF receptor (LIFR-α)-dependent cytokine signaling in DIO, we created an adipocyte-specific LIFR knockout mouse model (Adipoq-Cre;LIFR fl/fl ). Differentiated adipocytes derived from this model blocked LIF-induced triacylglycerol lipolysis. Adipoq-Cre;LIFR fl/fl mice on a high-fat diet (HFD) displayed reduced adipose STAT3 activation, 50% expansion in adipose, 20% body weight increase, and a 75% reduction in total hepatic triacylglycerides compared with controls. To demonstrate that LIFR-α signals adipocytes through STAT3, we also created an Adipoq-Cre;STAT3 fl/fl model that showed similar findings when fed a HFD as Adipoq-Cre;LIFR fl/fl mice. These findings establish the importance of obesity-associated LIFR-α/JAK/STAT3 inflammatory signaling in adipocytes, blocking further adipose expansion in DIO contributing to ectopic liver triacylglyceride accumulation.
Keywords: Animal Physiology; Biological Sciences; Cell Biology; Cellular Physiology; Endocrinology.
© 2021 The Authors.
Conflict of interest statement
T.G., A.G., J.Z.G., J.Y., A.Y.G., B.M.E., and P.I. acknowledge no conflicts of interest related to this work. Pfizer, Inc., is currently supporting a collaborative project with the R.E.I. laboratory that is independent of all data presented in this manuscript.
Figures


Similar articles
-
PPARα agonist WY-14,643 induces adipose atrophy and fails to blunt chronic ethanol-induced hepatic fat accumulation in mice lacking adipose FGFR1.Biochem Pharmacol. 2021 Oct;192:114678. doi: 10.1016/j.bcp.2021.114678. Epub 2021 Jul 13. Biochem Pharmacol. 2021. PMID: 34265279 Free PMC article.
-
Inhibition of Gastric Inhibitory Polypeptide Receptor Signaling in Adipose Tissue Reduces Insulin Resistance and Hepatic Steatosis in High-Fat Diet-Fed Mice.Diabetes. 2017 Apr;66(4):868-879. doi: 10.2337/db16-0758. Epub 2017 Jan 17. Diabetes. 2017. PMID: 28096257
-
Adipocyte-specific Hypoxia-inducible gene 2 promotes fat deposition and diet-induced insulin resistance.Mol Metab. 2016 Sep 28;5(12):1149-1161. doi: 10.1016/j.molmet.2016.09.009. eCollection 2016 Dec. Mol Metab. 2016. PMID: 27900258 Free PMC article.
-
Pdgfrα-Cre mediated knockout of the aryl hydrocarbon receptor protects mice from high-fat diet induced obesity and hepatic steatosis.PLoS One. 2020 Jul 30;15(7):e0236741. doi: 10.1371/journal.pone.0236741. eCollection 2020. PLoS One. 2020. PMID: 32730300 Free PMC article.
-
White Adipose Tissue Autophagy and Adipose-Liver Crosstalk Exacerbate Nonalcoholic Fatty Liver Disease in Mice.Cell Mol Gastroenterol Hepatol. 2021;12(5):1683-1699. doi: 10.1016/j.jcmgh.2021.07.008. Epub 2021 Jul 22. Cell Mol Gastroenterol Hepatol. 2021. PMID: 34303881 Free PMC article.
Cited by
-
The Molecular Basis and Therapeutic Potential of Leukemia Inhibitory Factor in Cancer Cachexia.Cancers (Basel). 2022 Jun 15;14(12):2955. doi: 10.3390/cancers14122955. Cancers (Basel). 2022. PMID: 35740622 Free PMC article. Review.
-
Phenome-Wide Association Study of Latent Autoimmune Diabetes from a Southern Mexican Population Implicates rs7305229 with Plasmatic Anti-Glutamic Acid Decarboxylase Autoantibody (GADA) Levels.Int J Mol Sci. 2024 Sep 21;25(18):10154. doi: 10.3390/ijms251810154. Int J Mol Sci. 2024. PMID: 39337639 Free PMC article.
-
New insights into Chlamydia pathogenesis: Role of leukemia inhibitory factor.Front Cell Infect Microbiol. 2022 Oct 18;12:1029178. doi: 10.3389/fcimb.2022.1029178. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36329823 Free PMC article. Review.
-
Cytokine-Mediated STAT3 Transcription Supports ATGL/CGI-58-Dependent Adipocyte Lipolysis in Cancer Cachexia.Front Oncol. 2022 Jun 15;12:841758. doi: 10.3389/fonc.2022.841758. eCollection 2022. Front Oncol. 2022. PMID: 35785158 Free PMC article.
-
Oncostatin M Induces Lipolysis and Suppresses Insulin Response in 3T3-L1 Adipocytes.Int J Mol Sci. 2022 Apr 23;23(9):4689. doi: 10.3390/ijms23094689. Int J Mol Sci. 2022. PMID: 35563078 Free PMC article.
References
-
- Arner P. Relationship between intracellular cyclic AMP and lipolysis in human adipose tissue. Acta Med. Scand. 1976;200:179–186. - PubMed
-
- Auernhammer C.J., Melmed S. Leukemia-inhibitory factor-neuroimmune modulator of endocrine function. Endocr. Rev. 2000;21:313–345. - PubMed
-
- Bischoff S.C., Boirie Y., Cederholm T., Chourdakis M., Cuerda C., Delzenne N.M., Deutz N.E., Fouque D., Genton L., Gil C. Clinical nutrition; 2016. Towards a Multidisciplinary Approach to Understand and Manage Obesity and Related Diseases. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

