S95021, a novel selective and pan-neutralizing anti interferon alpha (IFN-α) monoclonal antibody as a candidate treatment for selected autoimmune rheumatic diseases
- PMID: 33748735
- PMCID: PMC7972961
- DOI: 10.1016/j.jtauto.2021.100093
S95021, a novel selective and pan-neutralizing anti interferon alpha (IFN-α) monoclonal antibody as a candidate treatment for selected autoimmune rheumatic diseases
Abstract
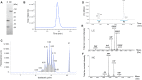
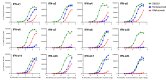
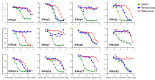
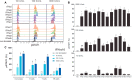
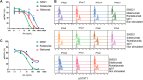
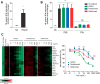
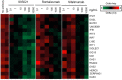
Increased interferon-α (IFN-α) production is a critical component in the pathophysiology of systemic lupus erythematosus (SLE) and other rheumatic autoimmune diseases. Herein, we report the characterization of S95021, a fully human IgG1 anti-IFN-α monoclonal antibody (mAb) as a novel therapeutic candidate for targeted patient populations. S95021 was expressed in CHOZN GS-/- cells, purified by chromatography and characterized by using electrophoresis, size exclusion chromatography and liquid chromatography-mass spectrometry. High purity S95021 was obtained as a monomeric entity comprising different charge variants mainly due to N-glycosylation. Surface plasmon resonance kinetics experiments showed strong association rates with all IFN-α subtypes and estimated KDs below picomolar values. Pan-IFN-α-binding properties were confirmed by immunoprecipitation assays and neutralization capacity with reporter HEK-Blue IFN-α/β cells. S95021 was IFN-α-selective and exhibited superior potency and broader neutralization profile when compared with the benchmark anti-IFN-α mAbs rontalizumab and sifalimumab. STAT-1 phosphorylation and the type I IFN gene signature induced in human peripheral blood mononuclear cells by recombinant IFN-α subtypes or plasmas from selected autoimmune patients were efficiently reduced by S95021 in a dose-dependent manner. Together, our results show that S95021 is a new potent, selective and pan IFN-α-neutralizing mAb. It is currently further evaluated as a valid therapeutic candidate in selected autoimmune diseases in which the IFN-α pro-inflammatory pathway is dysregulated.
Keywords: Autoimmune diseases; Biological therapy; Interferon-alpha; Primary Sjögren syndrome; Systemic lupus erythematosus.
© 2021 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: The study was supported by ImmunoQure AG and grants PUT1367 and 391 PRG377 from the 10.13039/501100002301Estonian Research Council to Dr. Kisand and Dr. Haljasmägi. Dr. Kisand and Dr. Hayday have a patent US20190071499A1 pending to Les laboratoires Servier, and Dr. Hayday reports personal fees from ImmunoQure outside the submitted work.
Figures

References
-
- Crow M.K., Olferiev M., Kirou K.A. Type I interferons in autoimmune disease. Annu. Rev. Pathol. 2019;14:369–393. - PubMed
-
- Novick D., Cohen B., Rubinstein M. The human interferon α/β receptor: characterization and molecular cloning. Cell. 1994;77:391–400. - PubMed
-
- Streuli M., Nagata S., Weissmann C. At least three human type alpha interferons: structure of alpha 2. Science. 1980;209:1343–1347. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

