Stimuli-Responsive Templated Polymer as a Target Receptor for a Conformation-based Electrochemical Sensing Platform
- PMID: 33748761
- PMCID: PMC7971449
- DOI: 10.1021/acsapm.0c01120
Stimuli-Responsive Templated Polymer as a Target Receptor for a Conformation-based Electrochemical Sensing Platform
Abstract
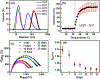
The use of highly crosslinked molecularly imprinted polymers as a synthetic target receptor has the limitations of restricted accessibility to the binding sites resulting in slow response time. Moreover, such artificial receptors often require additional transduction mechanisms to translate target binding events into measurable signals. Here, we propose the development of a single-chain stimuli-responsive templated polymer, without using any covalent interchain crosslinkers, as a target recognition element. The synthesized polymer chain exhibits preferential binding with the target molecule with which the polymer is templated. Moreover, upon specific target recognition, the polymer undergoes conformation change induced by its particular stimuli responsiveness, namely the target binding event. Such templated single-chain polymers can be attached to the electrode surface to implement a label-free electrochemical sensing platform. A target analyte, 4-nitrophenol (4-NP), was used as a template to synthesize a poly-N-isopropylacrylamide (PNIPAM)-based copolymer chain which was anchored to the electrode to be used as a selective receptor for 4-NP. The electrode surface chemistry analysis and the electrochemical impedance study reveal that the polymer concentration, the interchain interactions, and the Hofmeister effect play a major role in influencing the rate of polymer grafting as well as the morphology of the polymers grafted to the electrode. We also show that the specific binding between 4-NP and the copolymer results in a substantial change in the charge transfer kinetics at the electrode signifying the polymer conformation change.
Keywords: Conformation Change; Electrochemical Sensing; Molecular Imprinting; Nitrophenol; Poly(N-isopropylacrylamide); RAFT; Stimuli-Responsive; Templated Polymer.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
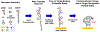


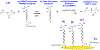



References
-
- Ye L; Mosbach K Molecular Imprinting: Synthetic Materials As Substitutes for Biological Antibodies and Receptors. Chem. Mater 2008, 20 (3), 859–868. 10.1021/cm703190w. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

