Distinct subsets of T cells and macrophages impact venous remodeling during arteriovenous fistula maturation
- PMID: 33748787
- PMCID: PMC7971420
- DOI: 10.1016/j.jvssci.2020.07.005
Distinct subsets of T cells and macrophages impact venous remodeling during arteriovenous fistula maturation
Abstract
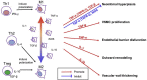
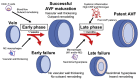
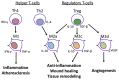
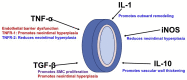
Patients with end-stage renal failure depend on hemodialysis indefinitely without renal transplantation, requiring a long-term patent vascular access. While the arteriovenous fistula (AVF) remains the preferred vascular access for hemodialysis because of its longer patency and fewer complications compared with other vascular accesses, the primary patency of AVF is only 50-60%, presenting a clinical need for improvement. AVF mature by developing a thickened vascular wall and increased diameter to adapt to arterial blood pressure and flow volume. Inflammation plays a critical role during vascular remodeling and fistula maturation; increased shear stress triggers infiltration of T-cells and macrophages that initiate inflammation, with involvement of several different subsets of T-cells and macrophages. We review the literature describing distinct roles of the various subsets of T-cells and macrophages during vascular remodeling. Immunosuppression with sirolimus or prednisolone reduces neointimal hyperplasia during AVF maturation, suggesting novel approaches to enhance vascular remodeling. However, M2 macrophages and CD4+ T-cells play essential roles during AVF maturation, suggesting that total immunosuppression may suppress adaptive vascular remodeling. Therefore it is likely that regulation of inflammation during fistula maturation will require a balanced approach to coordinate the various inflammatory cell subsets. Advances in immunosuppressive drug development and delivery systems may allow for more targeted regulation of inflammation to improve vascular remodeling and enhance AVF maturation.
Keywords: Arteriovenous fistula; Inflammation; Macrophages; T-cells; Vascular remodeling.
Figures
References
-
- Disbrow D.E., Cull D.L., Carsten C.G., 3rd, Yang S.K., Johnson B.L., Keahey G.P. Comparison of arteriovenous fistulas and arteriovenous grafts in patients with favorable vascular anatomy and equivalent access to health care: is a reappraisal of the Fistula First Initiative indicated? J Am Coll Surg. 2013;216:679–685. discussion: 685-6. - PubMed
-
- Christiansen J.F., Hartwig D., Bechtel J.F., Kluter H., Sievers H., Schonbeck U. Diseased vein grafts express elevated inflammatory cytokine levels compared with atherosclerotic coronary arteries. Ann Thorac Surg. 2004;77:1575–1579. - PubMed
-
- Kaygin M.A., Halici U., Aydin A., Dag O., Binici D.N., Limandal H.K. The relationship between arteriovenous fistula success and inflammation. Ren Fail. 2013;35:1085–1088. - PubMed
-
- Parma L., Baganha F., Quax P.H.A., de Vries M.R. Plaque angiogenesis and intraplaque hemorrhage in atherosclerosis. Eur J Pharmacol. 2017;816:107–115. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

