Long-term persistence of RBD+ memory B cells encoding neutralizing antibodies in SARS-CoV-2 infection
- PMID: 33748788
- PMCID: PMC7955929
- DOI: 10.1016/j.xcrm.2021.100228
Long-term persistence of RBD+ memory B cells encoding neutralizing antibodies in SARS-CoV-2 infection
Abstract
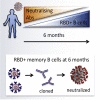
Considerable concerns relating to the duration of protective immunity against severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) exist, with evidence of antibody titers declining rapidly after infection and reports of reinfection. Here, we monitor the antibody responses against SARS-CoV-2 receptor-binding domain (RBD) for up to 6 months after infection. While antibody titers are maintained, ∼13% of the cohort's neutralizing responses return to background. However, encouragingly, in a selected subset of 13 participants, 12 have detectable RBD-specific memory B cells and these generally are increasing out to 6 months. Furthermore, we are able to generate monoclonal antibodies with SARS-CoV-2 neutralizing capacity from these memory B cells. Overall, our study suggests that the loss of neutralizing antibodies in plasma may be countered by the maintenance of neutralizing capacity in the memory B cell repertoire.
Keywords: COSIN; COVID; RBD; RBD tetramer; SARS-CoV-2; SARS-CoV-2 pseudovirus; functional MBCs; longitudinal tracking; memory B cells; neutralizing antibodies.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

