Protective low-avidity anti-tumour CD8+ T cells are selectively attenuated by regulatory T cells
- PMID: 33748824
- PMCID: PMC7958313
- DOI: 10.1093/immadv/ltaa001
Protective low-avidity anti-tumour CD8+ T cells are selectively attenuated by regulatory T cells
Abstract
Objectives: Regulatory T cells (Treg) play a major role in the suppression of protective anti-tumour T cell responses. In the CT26 BALB/c murine model of colorectal carcinoma, Tregs differentially suppress responses to two characterised CD8+ T epitopes, AH1 and GSW11, which results in an absence of detectable IFN-γ-producing GSW11-specific T cells in the spleen and lymph nodes of tumour challenged mice. Activation of GSW11-specific T cells correlates with protection against tumour progression. We wanted to examine the presence of non-functional GSW11-specific T cells in Treg replete and depleted mice, assess their phenotype and their affinity compared to AH1-specific T cells.
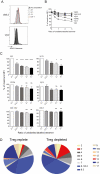
Methods: We used peptide-specific tetramers to identify tumour-specific CD8+ T cells and assessed the cell surface expression of markers associated with exhaustion (PD-1, Tim3 and Lag-3) and their function by IFN-g production using flow cytometry. We also assessed the T cell receptor (TcR) clonality of tumour-specific T cells. Tetramer competition assays were performed to determine the relative affinity of identified TcR.
Results: Here, we show that GSW11-specific T cells are in fact induced in Treg-replete, CT26-bearing mice, where they make up the majority of tumour-infiltrating CD8+ lymphocytes, but exhibit an 'exhausted' phenotype. This dysfunctional phenotype is induced early in the anti-tumour response in tumours. Depletion of Tregs prior to tumour challenge correlates with an altered T cell receptor (TcR) repertoire. Moreover, the avidity of GSW11-specific TcRs that expanded in the absence of Tregs was significantly lower compared with TcRs of CD8+populations that were diminished in protective anti-tumour responses.
Conclusion: Our results indicate that Tregs suppress the induction of protective anti-tumour T cell responses and may signify that low-avidity T cells play an important role in this protection.
Keywords: antigen presentation; immunotherapy; regulatory T cell; tumour immunity.
© The Author(s) 2020. Published by Oxford University Press on behalf of the British Society for Immunology.
Figures




References
-
- Naito Y, Saito K, Shiiba K et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res 1998;58(16):3491–4. - PubMed
-
- Nakano O, Sato M, Naito Y et al. Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res 2001;61(13):5132–6. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
