Secretory products of the corpus luteum and preeclampsia
- PMID: 33748839
- PMCID: PMC8222764
- DOI: 10.1093/humupd/dmab003
Secretory products of the corpus luteum and preeclampsia
Abstract
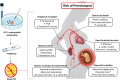
Background: Despite significant advances in our understanding of the pathophysiology of preeclampsia (PE), there are still many unknowns and controversies in the field. Women undergoing frozen-thawed embryo transfer (FET) to a hormonally prepared endometrium have been found to have an unexpected increased risk of PE compared to women who receive embryos in a natural FET cycle. The differences in risk have been hypothesized to be related to the absence or presence of a functioning corpus luteum (CL).
Objective and rationale: To evaluate the literature on secretory products of the CL that could be essential for a healthy pregnancy and could reduce the risk of PE in the setting of FET.
Search methods: For this review, pertinent studies were searched in PubMed/Medline (updated June 2020) using common keywords applied in the field of assisted reproductive technologies, CL physiology and preeclampsia. We also screened the complete list of references in recent publications in English (both animal and human studies) on the topics investigated. Given the design of this work as a narrative review, no formal criteria for study selection or appraisal were utilized.
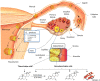
Outcomes: The CL is a major source of multiple factors regulating reproduction. Progesterone, estradiol, relaxin and vasoactive and angiogenic substances produced by the CL have important roles in regulating its functional lifespan and are also secreted into the circulation to act remotely during early stages of pregnancy. Beyond the known actions of progesterone and estradiol on the uterus in early pregnancy, their metabolites have angiogenic properties that may optimize implantation and placentation. Serum levels of relaxin are almost undetectable in pregnant women without a CL, which precludes some maternal cardiovascular and renal adaptations to early pregnancy. We suggest that an imbalance in steroid hormones and their metabolites and polypeptides influencing early physiologic processes such as decidualization, implantation, angiogenesis and maternal haemodynamics could contribute to the increased PE risk among women undergoing programmed FET cycles.
Wider implications: A better understanding of the critical roles of the secretory products of the CL during early pregnancy holds the promise of improving the efficacy and safety of ART based on programmed FET cycles.
Keywords: angiogenesis; corpus luteum; estradiol; estradiol metabolites; frozen-thawed embryo transfer; implantation; placentation; preeclampsia; progesterone; relaxin.
© The Author(s) 2021. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures

References
-
- Abbassi-Ghanavati M, Greer LG, Cunningham FG.. Pregnancy and laboratory studies: a reference table for clinicians. Obstet Gynecol 2009;114:1326–1331. - PubMed
-
- Abu-Musa A, Hannoun A, Khalil A, Masaad Z, Karam K.. Artificial endometrial preparation for oocyte donation using synthetic oestrogen and progestogen. Clin Exp Obstet Gynecol 1998;3:83–85. - PubMed
-
- ACOG. ACOG practice bulletin no. 202: gestational hypertension and preeclampsia. Obstet Gynecol 2019;1:e1–e25. - PubMed
-
- Akolekar R, Syngelaki A, Poon L, Wright D, Nicolaides KH.. Competing risks model in early screening for preeclampsia by biophysical and biochemical markers. Foetal Diagn Ther 2013;33:8–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

