Herbivore-induced plant volatiles mediate defense regulation in maize leaves but not in maize roots
- PMID: 33748996
- PMCID: PMC8360093
- DOI: 10.1111/pce.14052
Herbivore-induced plant volatiles mediate defense regulation in maize leaves but not in maize roots
Abstract
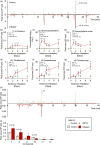
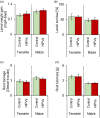
Plant leaves that are exposed to herbivore-induced plant volatiles (HIPVs) respond by increasing their defenses, a phenomenon referred to as priming. Whether this phenomenon also occurs in the roots is unknown. Using maize plants, Zea mays, whose leaves respond strongly to leaf HIPVs, we measured the impact of belowground HIPVs, emanating from roots infested by the banded cucumber beetle, Diabrotica balteata, on constitutive and herbivore-induced levels of defense-related gene expression, phytohormones, volatile and non-volatile primary and secondary metabolites, growth and herbivore resistance in roots of neighbouring plants. HIPV exposure did not increase constitutive or induced levels of any of the measured root traits. Furthermore, HIPV exposure did not reduce the performance or survival of D. balteata on maize or its ancestor teosinte. Cross-exposure experiments between HIPVs from roots and leaves revealed that maize roots, in contrast to maize leaves, neither emit nor respond strongly to defense-regulating HIPVs. Together, these results demonstrate that volatile-mediated defense regulation is restricted to the leaves of maize. This finding is in line with the lower diffusibility of volatiles in the soil and the availability of other, potentially more efficient, information conduits below ground.
Keywords: belowground plant-herbivore interactions; plant-plant interactions; priming; root defenses.
© 2021 The Authors. Plant, Cell & Environment published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare having no conflict of interest.
Figures



Similar articles
-
Plant Bio-Wars: Maize Protein Networks Reveal Tissue-Specific Defense Strategies in Response to a Root Herbivore.J Chem Ecol. 2018 Aug;44(7-8):727-745. doi: 10.1007/s10886-018-0972-y. Epub 2018 Jun 21. J Chem Ecol. 2018. PMID: 29926336
-
Volatiles from Low R: FR-Treated Maize Plants Increase the Emission of Herbivore-Induced Plant Volatiles in their Neighbors.J Chem Ecol. 2025 Jun 25;51(4):69. doi: 10.1007/s10886-025-01613-2. J Chem Ecol. 2025. PMID: 40560321 Free PMC article.
-
Defense Suppression through Interplant Communication Depends on the Attacking Herbivore Species.J Chem Ecol. 2021 Dec;47(12):1049-1061. doi: 10.1007/s10886-021-01314-6. Epub 2021 Sep 20. J Chem Ecol. 2021. PMID: 34541611 Free PMC article.
-
Plant volatiles as regulators of plant defense and herbivore immunity: molecular mechanisms and unanswered questions.Curr Opin Insect Sci. 2021 Apr;44:82-88. doi: 10.1016/j.cois.2021.03.010. Epub 2021 Apr 22. Curr Opin Insect Sci. 2021. PMID: 33894408 Review.
-
Induced immunity against belowground insect herbivores- activation of defenses in the absence of a jasmonate burst.J Chem Ecol. 2012 Jun;38(6):629-40. doi: 10.1007/s10886-012-0107-9. Epub 2012 Apr 12. J Chem Ecol. 2012. PMID: 22527052 Review.
Cited by
-
Effects of Phytophthora Inoculations on Photosynthetic Behaviour and Induced Defence Responses of Plant Volatiles in Field-Grown Hybrid Poplar Tolerant to Bark Canker Disease.J Fungi (Basel). 2021 Nov 15;7(11):969. doi: 10.3390/jof7110969. J Fungi (Basel). 2021. PMID: 34829256 Free PMC article.
-
Eco-evolutionary factors contribute to chemodiversity in aboveground and belowground cucurbit herbivore-induced plant volatiles.Plant Biol (Stuttg). 2025 Aug;27(5):847-860. doi: 10.1111/plb.13709. Epub 2024 Aug 20. Plant Biol (Stuttg). 2025. PMID: 39162182 Free PMC article.
-
Volatile organic compounds shape belowground plant-fungi interactions.Front Plant Sci. 2022 Dec 6;13:1046685. doi: 10.3389/fpls.2022.1046685. eCollection 2022. Front Plant Sci. 2022. PMID: 36561453 Free PMC article. Review.
-
Volatiles Emission by Crotalaria nitens after Insect Attack.Molecules. 2021 Nov 17;26(22):6941. doi: 10.3390/molecules26226941. Molecules. 2021. PMID: 34834034 Free PMC article.
References
-
- Arimura, G.‐i., Ozawa, R., Horiuchi, J. I., Nishioka, T., & Takabayashi, J. (2001). Plant–plant interactions mediated by volatiles emitted from plants infested by spider mites. Biochemical Systematics and Ecology, 29(10), 1049–1061. 10.1016/S0305-1978(01)00049-7 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

