Mechanical stimulation enhances development of scaffold-free, 3D-printed, engineered heart tissue grafts
- PMID: 33749089
- PMCID: PMC8900208
- DOI: 10.1002/term.3188
Mechanical stimulation enhances development of scaffold-free, 3D-printed, engineered heart tissue grafts
Abstract
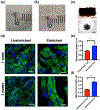
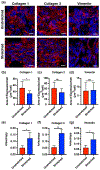
Current efforts to engineer a clinically relevant tissue graft from human-induced pluripotent stem cells (hiPSCs) have relied on the addition or utilization of external scaffolding material. However, any imbalance in the interactions between embedded cells and their surroundings may hinder the success of the resulting tissue graft. Therefore, the goal of our study was to create scaffold-free, 3D-printed cardiac tissue grafts from hiPSC-derived cardiomyocytes (CMs), and to evaluate whether or not mechanical stimulation would result in improved graft maturation. To explore this, we used a 3D bioprinter to produce scaffold-free cardiac tissue grafts from hiPSC-derived CM cell spheroids. Static mechanical stretching of these grafts significantly increased sarcomere length compared to unstimulated free-floating tissues, as determined by immunofluorescent image analysis. Stretched tissue was found to have decreased elastic modulus, increased maximal contractile force, and increased alignment of formed extracellular matrix, as expected in a functionally maturing tissue graft. Additionally, stretched tissues had upregulated expression of cardiac-specific gene transcripts, consistent with increased cardiac-like cellular identity. Finally, analysis of extracellular matrix organization in stretched grafts suggests improved remodeling by embedded cardiac fibroblasts. Taken together, our results suggest that mechanical stretching stimulates hiPSC-derived CMs in a 3D-printed, scaffold-free tissue graft to develop mature cardiac material structuring and cellular fates. Our work highlights the critical role of mechanical conditioning as an important engineering strategy toward developing clinically applicable, scaffold-free human cardiac tissue grafts.
Keywords: engineered heart tissue; human-induced pluripotent stem cells (hiPSCs); maturation; mechanical microenvironment; tissue engineering.
© 2021 John Wiley & Sons Ltd.
Conflict of interest statement
CONFLICT OF INTERESTS
The author(s) declare no competing interests.
Figures


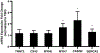
Similar articles
-
Maturation of human embryonic stem cell-derived cardiomyocytes (hESC-CMs) in 3D collagen matrix: Effects of niche cell supplementation and mechanical stimulation.Acta Biomater. 2017 Feb;49:204-217. doi: 10.1016/j.actbio.2016.11.058. Epub 2016 Nov 24. Acta Biomater. 2017. PMID: 27890729
-
3D-Printed Myocardium-Specific Structure Enhances Maturation and Therapeutic Efficacy of Engineered Heart Tissue in Myocardial Infarction.Adv Sci (Weinh). 2025 Mar;12(10):e2409871. doi: 10.1002/advs.202409871. Epub 2025 Jan 22. Adv Sci (Weinh). 2025. PMID: 39840547 Free PMC article.
-
Myocardial Tissue Engineering With Cells Derived From Human-Induced Pluripotent Stem Cells and a Native-Like, High-Resolution, 3-Dimensionally Printed Scaffold.Circ Res. 2017 Apr 14;120(8):1318-1325. doi: 10.1161/CIRCRESAHA.116.310277. Epub 2017 Jan 9. Circ Res. 2017. PMID: 28069694 Free PMC article.
-
Three-dimensional (3D) printed scaffold and material selection for bone repair.Acta Biomater. 2019 Jan 15;84:16-33. doi: 10.1016/j.actbio.2018.11.039. Epub 2018 Nov 24. Acta Biomater. 2019. PMID: 30481607 Review.
-
Scaffold-based and scaffold-free cardiac constructs for drug testing.Biofabrication. 2021 Jul 22;13(4). doi: 10.1088/1758-5090/ac1257. Biofabrication. 2021. PMID: 34233316 Review.
Cited by
-
The development, use, and challenges of electromechanical tissue stimulation systems.Artif Organs. 2024 Sep;48(9):943-960. doi: 10.1111/aor.14808. Epub 2024 Jun 18. Artif Organs. 2024. PMID: 38887912 Free PMC article. Review.
-
Application of medical imaging methods and artificial intelligence in tissue engineering and organ-on-a-chip.Front Bioeng Biotechnol. 2022 Sep 12;10:985692. doi: 10.3389/fbioe.2022.985692. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36172022 Free PMC article. Review.
-
Biological Scaffolds for Congenital Heart Disease.Bioengineering (Basel). 2023 Jan 2;10(1):57. doi: 10.3390/bioengineering10010057. Bioengineering (Basel). 2023. PMID: 36671629 Free PMC article. Review.
-
Development and Application of 3D Bioprinted Scaffolds Supporting Induced Pluripotent Stem Cells.Biomed Res Int. 2021 Sep 13;2021:4910816. doi: 10.1155/2021/4910816. eCollection 2021. Biomed Res Int. 2021. PMID: 34552987 Free PMC article. Review.
-
Cardiac Tissue Bioprinting: Integrating Structure and Functions Through Biomimetic Design, Bioinks, and Stimulation.Gels. 2025 Jul 31;11(8):593. doi: 10.3390/gels11080593. Gels. 2025. PMID: 40868723 Free PMC article. Review.
References
-
- Bargehr J, Hofsteen P, Bhandari S, Gambardella L, Ong L, Iyer D, Sampaziotis F, Weinberger F, Martinson M, Bernard W, Figg N, Bennett M, Murry C, Sinha S (2017). 5728Human embryonic stem cell derived epicardial cells advance cardiomyocyte-based heart regeneration. European Heart Journal, 38(suppl_1), ehx493.5728. 10.1093/eurheartj/ehx493.5728 - DOI
-
- Bing OHL, Ngo HQ, Humphries DE, Robinson KG, Lucey EC, Carver W, Brooks WW, Conrad CH, Hayes JA, & Goldstein RH (1997). Localization of α(I) collagen mRNA in myocardium from the spontaneously hypertensive rat during the transition from compensated hypertrophy to failure. Journal of Molecular and Cellular Cardiology, 29(9), 2335–2344. 10.1006/jmcc.1997.0465 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

