Large- and Medium-sized Arteries Remaining in Transmural Scar Distal to Permanent Coronary Ligation Undergo Neointimal Hyperplasia and Inward Remodeling
- PMID: 33749360
- PMCID: PMC8091545
- DOI: 10.1369/00221554211004297
Large- and Medium-sized Arteries Remaining in Transmural Scar Distal to Permanent Coronary Ligation Undergo Neointimal Hyperplasia and Inward Remodeling
Abstract
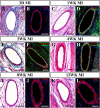
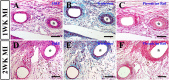
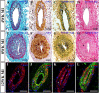
This study aimed to investigate the structural integrity and dynamic changes in chronically occluded residual arteries found in post-myocardial infarction (MI) scar. A transmural MI was induced in middle-aged, male Sprague-Dawley rats by left coronary artery ligation. The rats were euthanized 3 days and 1, 2, 4, 8, and 12 weeks after MI, and their hearts were processed into paraffin for histology, immunohistochemistry, and quantitative morphometry. It has been found that large- and medium-sized arteries were able to survive inside the transmural scars for 12 post-MI weeks. Furthermore, most residual arteries preserved their structural integrity for up to 2 weeks post-MI, but gradually all disused vessels had undergone neointimal hyperplasia and inward remodeling at later time periods. In addition, the replacement of vascular smooth muscle cells in the wall of residual arteries by extracellular matrix components led to a disruption of the vessel integrity and progressive obliteration of their lumen between 4 and 12 post-MI weeks. Taken together, this study demonstrate that residual arteries in post-infarcted region were capable of maintaining their structural integrity, including the patent lumen, during two post-MI weeks, suggesting that during this period they can be used as potential conduits for conceivable reflow of arterial blood within the scarred region of the heart.
Keywords: inward arterial remodeling; middle-aged rats; myocardial infarction; neointimal hyperplasia; residual coronary arteries; transmural scar.
Conflict of interest statement
Figures





References
-
- Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81(4):1161–72. - PubMed
-
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70(6):776–803. doi: 10.1016/j.jacc.2017.04.025. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

