Clinical Decision Support for Traumatic Brain Injury: Identifying a Framework for Practical Model-Based Intracranial Pressure Estimation at Multihour Timescales
- PMID: 33749613
- PMCID: PMC8077603
- DOI: 10.2196/23215
Clinical Decision Support for Traumatic Brain Injury: Identifying a Framework for Practical Model-Based Intracranial Pressure Estimation at Multihour Timescales
Abstract
Background: The clinical mitigation of intracranial hypertension due to traumatic brain injury requires timely knowledge of intracranial pressure to avoid secondary injury or death. Noninvasive intracranial pressure (nICP) estimation that operates sufficiently fast at multihour timescales and requires only common patient measurements is a desirable tool for clinical decision support and improving traumatic brain injury patient outcomes. However, existing model-based nICP estimation methods may be too slow or require data that are not easily obtained.
Objective: This work considers short- and real-time nICP estimation at multihour timescales based on arterial blood pressure (ABP) to better inform the ongoing development of practical models with commonly available data.
Methods: We assess and analyze the effects of two distinct pathways of model development, either by increasing physiological integration using a simple pressure estimation model, or by increasing physiological fidelity using a more complex model. Comparison of the model approaches is performed using a set of quantitative model validation criteria over hour-scale times applied to model nICP estimates in relation to observed ICP.
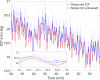
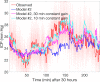
Results: The simple fully coupled estimation scheme based on windowed regression outperforms a more complex nICP model with prescribed intracranial inflow when pulsatile ABP inflow conditions are provided. We also show that the simple estimation data requirements can be reduced to 1-minute averaged ABP summary data under generic waveform representation.
Conclusions: Stronger performance of the simple bidirectional model indicates that feedback between the systemic vascular network and nICP estimation scheme is crucial for modeling over long intervals. However, simple model reduction to ABP-only dependence limits its utility in cases involving other brain injuries such as ischemic stroke and subarachnoid hemorrhage. Additional methodologies and considerations needed to overcome these limitations are illustrated and discussed.
Keywords: intracranial hypertension; intracranial pressure; patient-specific modeling; theoretical models; traumatic brain injury.
©J N Stroh, Tellen D Bennett, Vitaly Kheyfets, David Albers. Originally published in JMIR Medical Informatics (http://medinform.jmir.org), 22.03.2021.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures





Similar articles
-
Fully automated, real-time, calibration-free, continuous noninvasive estimation of intracranial pressure in children.J Neurosurg Pediatr. 2019 Aug 23;24(5):509-519. doi: 10.3171/2019.5.PEDS19178. Print 2019 Nov 1. J Neurosurg Pediatr. 2019. PMID: 31443086
-
Analysis of a Non-invasive Intracranial Pressure Monitoring Method in Patients with Traumatic Brain Injury.Acta Neurochir Suppl. 2018;126:107-110. doi: 10.1007/978-3-319-65798-1_23. Acta Neurochir Suppl. 2018. PMID: 29492543
-
Comparison of Different Calibration Methods in a Non-invasive ICP Assessment Model.Acta Neurochir Suppl. 2018;126:79-84. doi: 10.1007/978-3-319-65798-1_17. Acta Neurochir Suppl. 2018. PMID: 29492537
-
Clinical applications of a non-invasive ICP monitoring method.Eur J Ultrasound. 2002 Nov;16(1-2):37-45. doi: 10.1016/s0929-8266(02)00044-7. Eur J Ultrasound. 2002. PMID: 12470849 Review.
-
Advances in Intracranial Pressure Monitoring and Its Significance in Managing Traumatic Brain Injury.Int J Mol Sci. 2015 Dec 4;16(12):28979-97. doi: 10.3390/ijms161226146. Int J Mol Sci. 2015. PMID: 26690122 Free PMC article. Review.
Cited by
-
Intracranial pressure-flow relationships in traumatic brain injury patients expose gaps in the tenets of models and pressure-oriented management.medRxiv [Preprint]. 2024 Jan 18:2024.01.17.24301445. doi: 10.1101/2024.01.17.24301445. medRxiv. 2024. Update in: Front Physiol. 2024 Aug 12;15:1381127. doi: 10.3389/fphys.2024.1381127. PMID: 38293069 Free PMC article. Updated. Preprint.
-
The Role of Neuregulin-1 in Steatotic and Non-Steatotic Liver Transplantation from Brain-Dead Donors.Biomedicines. 2022 Apr 23;10(5):978. doi: 10.3390/biomedicines10050978. Biomedicines. 2022. PMID: 35625715 Free PMC article.
-
Intracranial pressure-flow relationships in traumatic brain injury patients expose gaps in the tenets of models and pressure-oriented management.Front Physiol. 2024 Aug 12;15:1381127. doi: 10.3389/fphys.2024.1381127. eCollection 2024. Front Physiol. 2024. PMID: 39189028 Free PMC article.
References
-
- Lassen NA. Cerebral blood flow and oxygen consumption in man. Physiol Rev. 1959 Apr 01;39(2):183–238. doi: 10.1152/physrev.1959.39.2.183. https://journals.physiology.org/doi/10.1152/physrev.1959.39.2.183?url_ve... - DOI - DOI - PubMed
-
- Armstead WM. Cerebral blood flow autoregulation and dysautoregulation. Anesthesiol Clin. 2016 Sep;34(3):465–77. doi: 10.1016/j.anclin.2016.04.002. http://europepmc.org/abstract/MED/27521192 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

